No time to read now?
-> Download the article as a handy pdf
List of contents
Data Product Information Management in the EU: XEVMPD, PMS, PLM and ePI in 2025
Martti Ahtola | Jun 20, 2025
The system was built a long time ago and has not seen significant updates in the past years. The use of the system requires Internet Explorer, a browser from Microsoft that has not existed in three years, and other components that are no longer used in today’s software.
Tepsivo is a pharmacovigilance company, but every now and then, drug safety activities border with processes that are often categorized as “regulatory affairs”. An example of this would be when product information needs to be updated in authority databases, or a marketing authorization application submission requires additional documents to be prepared.
This means that while the Tepsivo team does not necessarily work daily with product information management and with preparation of regulatory submissions, it happens fairly regularly, and we need to understand what is happening with the processes orchestrated by different authorities.
While one could assume that the regulatory landscape is fairly stagnant if Internet Explorer is still a requirement in the EU, currently the opposite is true. Recently the US FDA updated their submission gateway system, the Electronic Submission Gateway Next Generation (ESG NextGen) became available in 2025 and is ready to receive electronic submissions.
In the EU, EMA has been working with their SPOR update now for a few years, and while the work continues, there are already new systems and features in production and testing, and there are weekly updates on this topic.
In 2025 XEVMPD is still stuck in the past
It has been a while since I personally worked with XEVMPD, but last fall, to be exact on 10th of October 2024, I and many other registered XEVMPD users received an email from EMA explaining that an issue related to the unusually early time-out of sessions had been detected.
According to the email, the issue was related to a browser configuration and for that reason, EMA wanted to notify all XEVMPD users that they should amend their IE Tab extension settings, to ensure that Chrome/Edge cookies are not forwarded to IE Tab. With a minor update of unchecking the “Forward Chrome cookies to IE tab” option, things went back to “normal” again.
What really started my latest dive into the world of system development at EMA was that one of my colleagues started using XEVMPD for the first time and had some issues with the first login, so I was helping.
This got me thinking; What is the “normal” for XEVMPD users? I am currently not an active user myself, but back in 2021, I started my series of complaints about the fact that if you were using XEVMPD to make the legally required product information updates, you were required to use Internet Explorer (IE).
Now, in 2025, this is still the case, even though Internet Explorer has not existed since 2022 and based on my colleague’s experience, I would say that the biggest challenge for a new XEVMPD user is not the actual use of the system (the process is clear and the documentation is very thorough and easy to follow) but the hurdle of getting into the system with a modern computer.
Recap on XEVMPD
As it had been almost three years since my last blog post about the topic and I had not been actively monitoring the updates related to the system, I had to read through all four previous posts that I had written about the topic.
In my first blog post on the topic in 2021 I was just complaining that the Internet Explorer requirement was a blast from the past. Now I can only say that this is more true than ever. And still, there are important systems such as XEVMPD that are reliant on Internet Explorer.
Later in 2021 I was voicing concerns but I was still optimistic that EMA would do something about the XEVMPD’s Internet Explorer requirement before the retirement of the browser in 2022. Well, the retirement of IE on June 15th of 2022 went by and it has been three years since.
In early June of 2022, less than two weeks before the retirement of IE, I posted that Internet Explorer was coming to the end of its lifetime and EMA had given the industry almost no instructions on how to proceed with usage of XEVMPD.
What the EMA had at that point communicated was that there was a new ‘xEVMPD Support’ section available under ‘User Support’ in the EudraVigilance Restricted area. EMA added instructions on how to install, license, and use IE Tab extension on Edge or Chrome.
At that point during the summer of 2022 we entered the situation where we still are now three years later:
👉 IE Tab extension for modern browsers is required to run XEVMPD.
👉 Users of XEVMPD are able to acquire a license for IE Tab from the EudraVigilance page and the software license is paid by EMA.
👉 The extension emulates old versions of Internet Explorer software and according to the developer of the extension it can be used for Java, Silverlight and ActiveX.
In December 2022 I made the last post on XEVMPD reporting that it would not be updated, and with the assumption that it would be soon replaced by SPOR PMS.
Before writing that last blog post on the topic, I had asked EMA about the possible updates and additional browser support for XEVMPD and the response was:
“The XEVMPD/Article57 user interface (EVWEB) can only be accessed using an IE Tab extension in Google Chrome or Microsoft Edge. There are no plans to change the platform as XEVMPD submissions will be replaced with PMS submissions in the future. PMS UI will be built taking into account those limitations and using current browsers.”
Is it already time for PMS?
Now, over two years later, my colleagues are still using XEVMPD and struggling with the IE extension. Why? Wasn’t PMS already supposed to be up-and-running by now? PMS stands for Product Management Service, and it is the “P” in SPOR.
Before starting to write this blog post I wasn’t sure what the answer was, so as already mentioned, I did a little bit of research.
In short, there is a lot going on with PMS, but no, it still isn’t a time for PMS and we are still stuck with XEVMPD. How long until XEVMPD is retired? That part is unclear, but I was able to find out some new information on the matter.
Recap on PMS
In 2022, EMA was implementing the Product Data Management Services (PMS), a system designed to replace XEVMPD in management of product information and facilitating data exchange between regulators and industry. The PMS is part of the broader effort to implement ISO IDMP standards with Substances Management System SPOR, which aims to standardize the way pharmaceutical product information is managed.
Back in 2022, the plan was to replace the existing eXtended EudraVigilance Product Report Message (XEVPRM) XML format with the new ISO IDMP-compatible HL7 FHIR (XML) format.
The Digital Application Dataset Integration (DADI) project, later renamed the Product Lifecycle Management (PLM) Portal, was aimed at replacing the .pdf-based electronic application and variation forms with web-based forms. The PMS was linked to the DADI project as it would provide the necessary product data for the new electronic forms.
In 2022, EMA was still deciding on key aspects of the PMS implementation, such as the two-step approach for CAPs and non-CAPs and the timing of the XEVPRM replacement. The completion of the PLM Portal was expected by the end of 2023. If EMA had chosen to wait for the PLM Portal’s completion, the full implementation of the PMS would have been delayed until 2024 at the earliest.
Now it’s already Q2 of 2025. What is the situation with PMS and PLM Portal? At least XEVMPD is still running, meaning that the transition to PMS and PLM has not been fully completed.
If you enter the SPOR website, the other sections of the four-letter abbreviation of SPOR already work, besides the “P” for products that would lead you to PMS.
That being said, also the Substances Management Services (SMS) only kind of works. According to the SMS site “SMS is currently live but not yet available in the SPOR Portal.” External users can access the SMS data in EUTCT or IRIS to view and search substance data or download it as a CSV file. For any change requests the user has to contact the EMA Service Desk.
According to the On-boarding of users to SPOR data services guidance document PMS is not yet publicly accessible. A limited data set of PMS will be made publicly accessible via application programming interface (API) and Product User Interface (PUI). To access PUI you should follow the PUI guidance documents.
PLM Portal
The PLM Portal contains three main sections:
1) Electronic application forms (eAF)
2) Electronic product information (ePI)
3) Product Management Service (PMS)
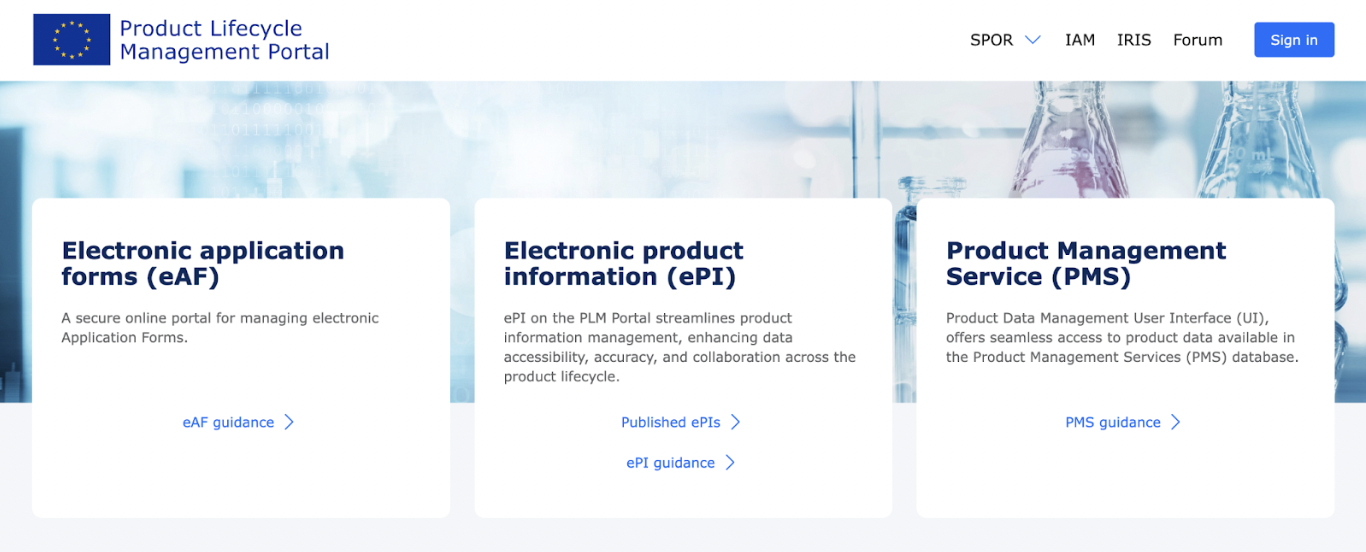
Screenshot from the PLM Portal website
Below is more information about the different parts of the PLM Portal.
PMS News
The PMS news section contains a lot of information about recent updates on recent developments to PMS.

Screenshot from the PMS News Articles feed
Based on the number of news just on the PMS, one would expect that a lot is happening. And that seems to be at least partially the case.
Here are some example news:
🗞️ The data load of non-Centrally Authorized Product (non-CAP) information on the PMS Product User Interface has been successfully completed in September 2024. All human non-CAP and CAP data is available in read-only mode in PLM Portal, and as mentioned in a separate news piece, this means that the product information is also available in the web-based eAF.
🗞️ Also from September onwards, the PMS API, launched on 3 July 2024 in read-only mode for industry users only, is now available for NCAs managing both human and veterinary medicinal products. Users from these NCAs are now able to view their non-Centrally Authorized Products (non-CAPs) (as well as CAPs) ISO-IDMP compliant human product data in the API.
🗞️ From Q1 2025, EMA will launch write access for MAHs through the PMS Product User Interface (PUI) first and API later. This functionality will be crucial for shortages monitoring via the European Shortages Monitoring Platform (ESMP). This news piece asks MAHs to prepare for this by already adding the necessary information through XEVMPD now, and then updating the required information in PMS once the fields become editable in PMS during Q1 2025.
🗞️ There should be a new updated version (previous version published in 2022) of the EU IDMP Implementation Guide (IG) on “Implementation of International Organization for Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe” available by the end of 2024.
Currently, there are no release notes available for PMS yet. The PMS guidance section contains information about the current version of the EU IDMP Implementation Guide, PUI guidance documents, guidance on onboarding users, FAQs on PMS, links to past training sessions and information on the union list of critical medicines.
PMS Roadmap and new EMA page for PMS
According to the recently published updated PMS roadmap, the use of XEVMP by MAHs to review and maintain CAPs and non-CAP data, at least to support other projects such as pack sizes, enriching data on NAPs or pending MRPs / DCPs will continue to 2026 and beyond.
It should be noted that from Q3 onwards the enrichment for non-CAPs using PMS API should start for MAHs as well.
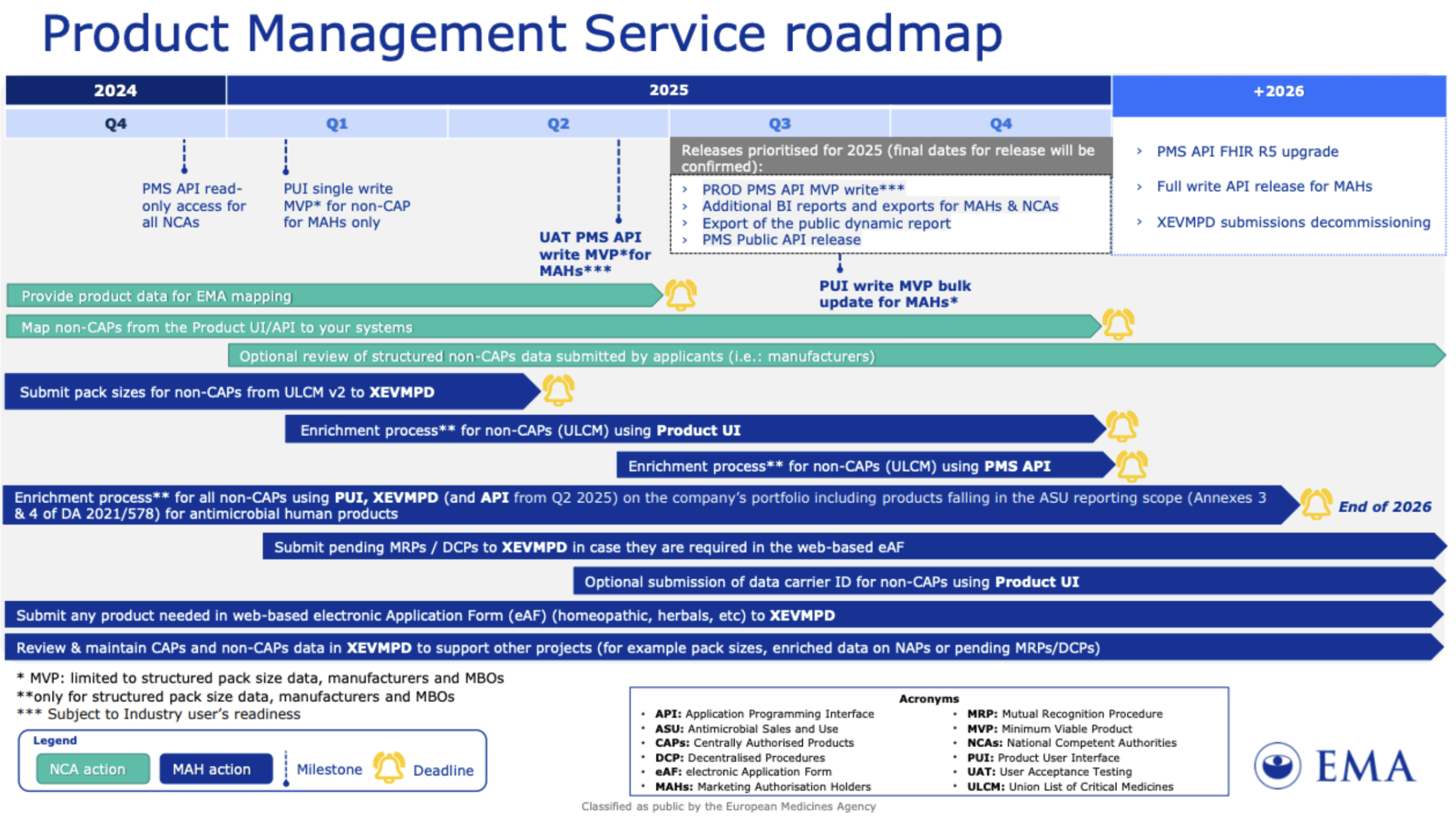
Recently updated PMS Roadmap
The new EMA page for PMS contains information about the implementation in an easier format, aimed at non-technical users.
You can for example learn that EMA completed “Product data preparatory” phase in March 2024 by launching the referentials management service (RMS) and organisation management service (OMS) and by migrating (product) data from existing systems like SIAMED and XEVMPD to PMS.
Electronic Product Information (ePI) for Human Medicines
According to the PLM Portal, “ePI on the PLM Portal streamlines product information management, enhancing data accessibility, accuracy, and collaboration across the product lifecycle.”
The published ePIs section on the website can be used to search and browse ePI of authorised PI published in the context of the ePI pilot project. Currently it contains 23 rows of products that can be downloaded as an XLSX file. These ePIs were created during the pilot that was finalized last year and results were published in January 2025.
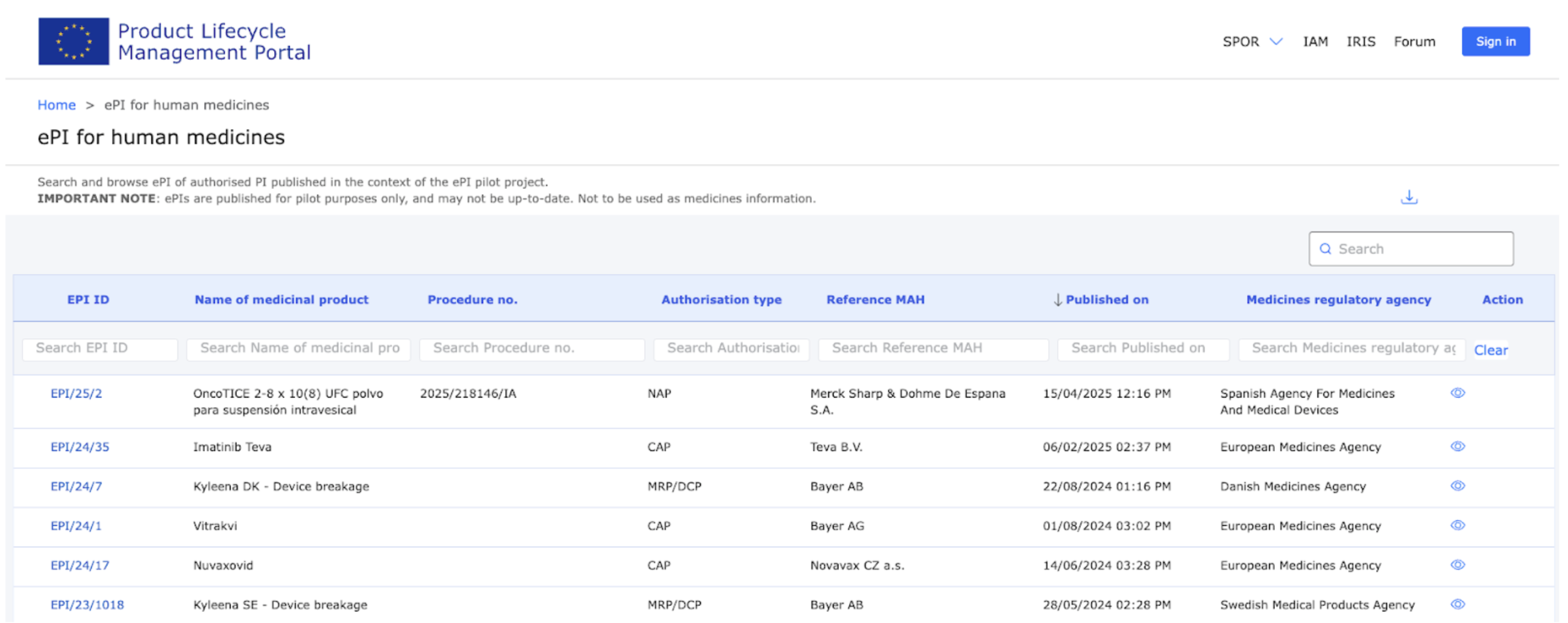
The screenshot shows few of the pilot products available in ePI during the spring of 2025
It is possible to open the records and view SmPC, Labelling and PIL for the products through the website as shown below.

The screenshot shows example electronic product information available in ePI system
The ePI section of the PML Portal contains also guidance for implementing the electronic product information. These implementation guidance documents have been updated as recently as April 2025.
ePI Pilot Report
The ePI pilot report summarizes the experience of creating ePIs during regulatory procedures for EU human medicines. According to the pilot report the aim of ePI is to benefit patients and healthcare professionals by providing up-to-date, accessible, multilingual product information at the point of need.
The pilot ran from July 2023 to August 2024, involving EMA and NCAs of Spain, Denmark, the Netherlands, and Sweden.
Real regulatory procedures were used to create and publish ePIs.
Key Performance Indicators set for the pilot were mostly met:
🎯 Time to create ePI averaged 5.2 hours (target 8 hours)
🎯 92% of co-opted procedures had ePIs created/submitted
🎯 90% of submitted ePIs for positive outcome procedures were published
🎯 Over 80% of participants found business processes viable.
🎯 Editor usability partially met targets.
Main recommendations included improving guidance (e.g., for non-QRD templates, translations, images), business processes (covering all procedure types, linguistic review, reminders on procedure numbers, integration into procedure management systems), and PLM portal enhancements (styling separation, FHIR import API, improved labeling, quality control export to Word).
The ePI pilot concluded no blocking issues preventing ePI integration into regulatory processes as an add-on. Implementation will start voluntarily for CAPs and roll out phased to NCAs. Post-implementation will focus on extending digital workflows, improving functionalities, broadening product scope, and supporting stakeholder engagement and change management.
The pilot report emphasizes patient access solutions for electronic package leaflets alongside printed copies for successful adoption.
According to the pilot report the ePI editor requires improvements especially in handling images, tables, and styling from pasted Word content.
Future planned functionalities include ePI versioning, linking ePI to PMS, API improvements for data retrieval and upload, and support for parallel procedures.
ePI – Open for consultation: reflection paper on accessing ePI from medicine package
The ePI news section contains information about a reflection paper, currently open for public consultation, that outlines considerations for linking to ePI by scanning a code on the medicine package. Comments can be submitted before the end June 2025.
According to the ePI website, the goal is to provide patients, consumers, and healthcare professionals with direct access to the most current and relevant information on a medicine’s safety, benefits, and usage, ensuring that patients and healthcare professionals have the right information when needed.
The reflection paper outlines considerations for accessing ePI by scanning a code on the medicine package. The paper proposes that ePI, in particular the package leaflet, displayed in HTML format, should be available via straightforward scanning of the medicine package.
The paper suggests using the existing DataMatrix (2D code) on prescription medicine packaging, which contains data carrier identifiers like GTIN/NTIN/PPN for anti-falsification measures. The DataMatrix should be leveraged to link to the ePI without requiring additional codes to avoid packaging space constraints and user confusion.
Patients should be able to scan the DataMatrix using their phone to access the electronic package leaflet (ePL) easily and directly, without barriers (e.g., no login screens). The ePL should be presented primarily in HTML format for optimal accessibility and user experience and should be non-promotional and consistent with authorized product information.
Solutions should cover as many medicines as possible EU-wide to avoid the proliferation of multiple apps, which complicates access and disadvantages users with low digital literacy. ePI should support display in patients’ preferred language where available, with easy browsing between languages and customization of content targeting patients or healthcare professionals.
As expected, the reflection paper suggests that a robust governance structure is needed to maintain and provide reliable, secure, and up-to-date ePI. This includes linking ePI with the correct package data identifiers via systems like the PLM portal and adhering to standards such as ISO-compliant resolvers and EU IDMP Implementation Guide.
The paper discusses challenges such as coverage of over-the-counter medicines, parallel trade medicines, primary packaging, and concurrent ePI versions for cases like excipient changes.
Solutions with similar functionality have been implemented in Nordic countries, Japan, Spain, Germany, and an app developed for Ukrainian refugees in Poland.
SPOR Quarterly Update Webinars
In April, the EMA organized a SPOR and XEVMPD status update webinar. They are organizing quarterly SPOR updates and the next one in July 2025. The Q1 webinar contained a status update from the product owners and described the plan for the rest of the year.
One of the biggest updates is that EMA is currently going through IT supplier change. The knowledge transfer is ongoing and the change in development team is understandably slowing down the development.
The SPOR webinar gave an update on the different areas of SPOR. SMS, RMS and OMS are already used in different parts of the EMA systems. Obviously also XEVMPD is also used and according to the PMS product owner, there is no timeline to replace XEVMPD nor is there even a roadmap when this would happen.
SMS Updates
The substance management system SMS is going through continuous data enrichment activities. Currently the system contains 69 thousand substances out of which 40% has been reviewed by the EMA’s team.
According to the webinar SMS API access can be requested through EMA Account Management to get access to all SMS data real time. This was new information to me because this information is not easily available anywhere on the EMA website or even in the SPOR portal. According to the SPOR portal the users can use EUTCT or IRIS to view and search substance data or download the data as .csv files.
According to the webinar SMS will not be part of the SPOR portal in the future either, but it will be in IRIS. This would mean that out 2 out of 4 parts (substances and products) of the SPOR would be actually outside of the SPOR. Whether this has any real-life impact, it is hard to say.
At least for me the main point is that it will be possible to read and write information through the API so that we can integrate Tepsivo software with the EMA systems and this would mean that the Tepsivo users would not have to worry about where the data is available in EMA’s complicated web of services and sites either.
OMS Updates
According to the webinar, XEVMPD is currently not using data from OMS. According to my notes this was not further explained in the session, but I would guess that the reason is that the data of all product information in XEVMPD would need to be updated, or at least this would require a process update for the marketing authorization holders.
For OMS the API is already available and the guidance for registration and use is available in the OMS portal. However, it has to be said that the API guidance is anything but easy to follow.
The API Specifications document seems to start from the basics such as a Wikipedia explanation of API but is missing key information such as what is the actual URL of the API or how the authentication works in practice after you have the sufficient role from EMA Account Management.
But this type of thing should be familiar to anyone who has read EudraVigilance or XEVMPD guidelines that also do not contain links or even hints to how to access the systems.
RMS Updates
According to the RMS product owner who spoke in the webinar, for RMS the current improvement work includes the addition of EMDN, list of medical devices and addition of ATC translations during 2025.
Other upcoming features for RMS include MedDRA API implementation, most likely in relation to the recent addition of MedDRA API access, and veterinary variation classification.
The veterinary update is apparently related to a new legislation, however it was not further explained to which legislative update exactly. Maybe this is obvious to those who have been following the RMS updates and/or those who are following the progress of veterinary medicines legislation in the EU, but search through EMA’s website and Google search did not explain this.
XEVMPD Updates – New User Interface coming?
The inclusion of XEVMPD as one of the sections of the quarterly SPOR update webinar was a sign that XEVMPD is not going anywhere any time soon. It could have been interpreted differently, maybe something big is happening soon, but somehow the first option seems more likely.
One of the most interesting takeaways from the webinar was that there has been an assessment for the new XEVMPD user interface (UI). This is the user interface update that EMA told me in 2022 that they would not perform.
The goal of the UI update is that the users can access XEVMPD without the need for ActiveX or IE Tab extension to their browser. This would improve the user experience because then XEVMPD would be fully browser based and could be used with any modern browser.
The initial assessment for a technical solution was done during Q1 2025, and the XEVMPD team is requesting management approval for the implementation in Q2 2025 for the implementation of the suggested solution. If approved by the management team, the development of the new UI would start during Q3 2025.
The webinar contained one other interesting piece of information regarding the current connection between XEVMPD and PMS. XEVMPD does not use data from PMS. PMS is apparently synchronized with data from XEVMPD (more about this below).
PMS Updates – Write API in Testing
A lot of information related to the PMS was shared and it was clear from the webinar that this is the most important part of the SPOR and the part that is still most unfinished.
Write PMS API Implementation Guide V1 was published earlier this year to support MAH and software developers building connections between their product information management systems and the PMS API. According to the presentation there is also increased user support available.
Additional information helping with the implementation of the integration also includes a new version of the Chapter 2 Product Management Service (PMS) – Implementation of International Organization for Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe – Data elements for the electronic submission of information on medicinal products for human use.
The testing of the PMS write API has started during the spring 2025.
Unlocking Integration – PMS API Write
In March 2025 EMA organized another webinar, called Product Management Service (PMS) webinar : Unlocking Integration – MAH & Software Developers to explore PMS API Machine-to-Machine Connection.
While the webinar focused on the integration and API(s) of PMS, it contained a recap on the basics of the service for product data.
According to the webinar presentation, the goal of PMS is to be the single source of truth for trustworthy, enriched and validated data for medicinal products in the EU. This goal, which is one of the basic principles of building database software, should sound familiar to anyone working with data. There’s almost always a goal to have a single source of truth for data.
The PMS data model aligns with ISO IDMP standard for authorized medicinal products, but it only contains a subset of the ISO IDMP fields. While it is “only a subset”, it still includes more than 180 fields most of which are repeatable, meaning that another table is required in the database to hold the data for the sub-records. Many of these fields are linked to the lists provided in RMS.
As mentioned above the PMS team at EMA has been working on a new version of Chapter 2 of the EU implementation guide for the development of compliant systems. The Chapter 6 of the implementation guide instructs with the integration to PMS.
Accessing PMS Data
There are two ways to access the product data in PMS:
- PMS Product User Interface (PUI)
- PMS API
The product user interface has been available for viewing data since 2024 and now starting from January 2025 it has been possible to also update the PMS data through the system. So this is all fairly new.
As mentioned above, if you go through the SPOR Portal, you would probably find this information or you could even see outdated information as the main information is available elsewhere, in the PLM Portal, and there are a lot of updates coming in, so it takes a bit of time to delve into the topic.
Another way to view and update the data in PMS is through the API. The API is useful when there is a lot of product data that needs to be maintained, so big pharma and generic pharmaceutical companies. For these organizations it is much easier to maintain the product information in their internal database and integrate that system to the EMA database, using the API.
It has been possible to view the product data in the PMS using the read API since 2024 for registered marketing authorization holders and as mentioned in the quarterly SPOR update the testing of the write API is now commencing with a small number of volunteer organizations.
How PMS data will be used
According to the webinar slides the PMS data will be used to transition from paper-based submissions to electronic data submissions driving the shift towards full digitalisation. This refers to the replacement of XEVMPD submissions and their variations using the old pdf forms.
PMS will be integrated to the EU regulatory processes meaning that all pharmaceutical companies will be required to integrate PMS data, enabling the exchange of structured data and its reuse throughout the product lifecycle.
The PMS data will be partially public and there will be a public API that can be used by the general public without authorization.
As mentioned above, there will be a link between ePI and PMS.
The structured product data in PMS can potentially support European Health Data Space (EHDS) in the context of ePrescription and eDispensation.
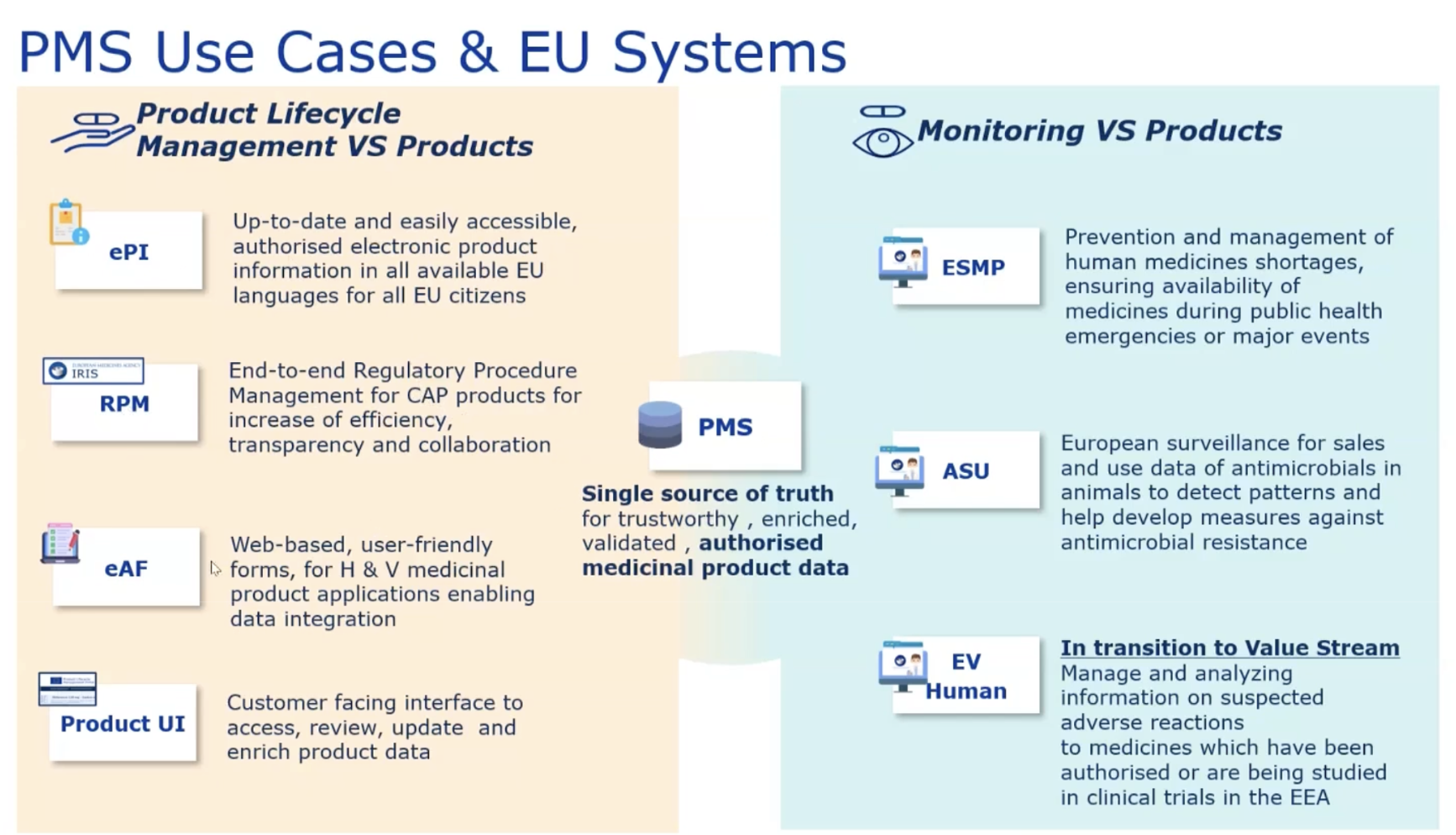
Slide depicting the use cases and EU systems that would use and update PMS data once it is fully available
PMS Write API
The goal for the PMS team at EMA is to have the PMS write API available in 2026. According to the webinar it is already possible to access the PMS API if you request the applicable roles in the EMA Account Management.
First, you should request IRIS/PLM Industry Admin role and then request PMS Industry API User role.
PMS Read API
The PMS read access has been available since July 2024 to all industry users and since September 2024 the access has been available to all users at national competent authorities responsible for human and veterinary medicine.
Users at those national competent authorities responsible for human medicinal products only, received read access in December 2024.
The next steps for PMS read API are the release of Public API and upgrade of the API to the FHIR R5 standard.
What is in PMS right now?
PMS contains authorized CAPs and non-CAPs data. PMS data is put together from a combination of the data available in SIAMED II (EMA’s internal database) and XEVMPD.
Because the PMS data model contains a large number of new fields compared to the XEVMPD/SIAMED attributes, there are currently about 80 fields that are empty. The MAHs need to fill in these empty fields.
What is coming next?
EMA’s team is promising example XMLs for the MAHs to use in their system development.
The industry representatives have been asking for a test / sandbox environment as it would be much easier to develop with a test tool instead of using only guidelines, but there is no test environment coming.
The PMS Write API should be fully in production in 2026.
Write API should be available already during Q3 2025 and by the end of 2025 MAHs should have enriched their data in PMS. The timeline for updating the missing fields was questioned by the industry representatives, due to the missing XML examples and test environment.
It should be noted that the data update required by the MAHs is for those 80 something fields that are not in XEVMPD. Rest of the data needs to be submitted through XEVMPD (non-CAPs) even after the PMS Write API becomes available.
In relation to a question about the Medicinal Product Identifier (MPID) field implementation to EudraVigilance, that have been part of the EU E2B R3 structure as described in EU Individual Case Safety Report (ICSR) Implementation Guide since 2021, the answer by EMA’s team was that MPID implementation for EV is beyond the scope of 2025. So it seems that there is no clear plan in place to implement these fields to EudraVigilance at least in the near future.
XEVMPD Decomissioning
The webinar presentation contained a PMS roadmap slide saying that XEVMPD will be decommissioned in “+2026”. This should be probably understood that there is an idea to decommission XEVMPD at some point in 2026 or later, because in the SPOR status update webinar it was said that there is not even a roadmap to replace XEVMPD.
However, it is safe to say that the goal still is to replace XEVMPD at some point.
Conclusion
There are a lot of things going on and many updates available, but it seems after the investigation that things are moving slowly compared to the original plan.
Despite being outdated and still reliant on Internet Explorer, XEVMPD is still in use, but PMS capabilities are becoming more and more available, so it is likely that XEVMPD is decommissioned some time during the latter half of 2026 or 2027. The plans to update XEVMPD user interface seem to indicate that the system would be running still in 2027.
Once the move from XEVMPD to PMS has happened, the management of product information should become much easier for all the stakeholders. The work around submission of product information and especially the work around product information updates.
There will not only be the benefit to the pharmaceutical companies and regulators, but the public will benefit as well with easier access to up-to-date product information thanks to the ePI. The public will also be able to access the product information through the public PMS API.
Did you like the article? Share with your network!
…or tell us your opinion.
Follow our newsletter!
Keep up with industry trends and get interesting reads like this one 1x per month into your inbox.
Learn more about Tepsivo
We deliver modern PV solutions to fulfill your regulatory needs using less resources. See how we do it >

0 Comments