No time to read now?
-> Download the article as a handy pdf
List of contents
Analyzing Compliance Language:
What Are GxP Requirements?
Written by Martti Ahtola | Dec 9, 2024

Introduction
The term GxP is frequently used to refer in a general way to a collection of quality guidelines. The Wikipedia definition is that “GxP is a general abbreviation for the “good practice” quality guidelines and regulations.” The “x” in GxP stands for the various fields, including the pharmaceutical and food industries, for example GCP is Good Clinical Practice and GVP is Good Pharmacovigilance Practice.
A “c” or “C” is sometimes added to the front of the initialism. The preceding “c” stands for “current.” For example, cGMP is an abbreviation for “current good manufacturing practice”.
The GxP requirements are often more well-known in the pharmaceutical industry than the legal requirements. And for a good reason. When looking at the medicinal product legislation for example in the European Union (EU) or the United States (US), it is clear that Good Manufacturing Practice (GMP), Good Distribution Practice (GDP), and Good Clinical Practice (GCP) are part of the legislative documents.
Meanwhile the EU legislation only briefly mentions the Good Pharmacovigilance Practice (GVP) and the GVP documents contain parts that are legal requirements and parts that are suggestions how to implement the legislation to practice. In the US the approach to GVP is even more limited.
In this blog text, we’ll look into the EU legal text related to GVP and compare it to the other pharmaceutical Good “Something” Practices. We look at what are the legal requirements related to these guidelines and how those legal requirements are described in the related legislation and how they are shown in the guidelines themselves.
Quick AI summary of the blog post
The term GxP encompasses a collection of quality guidelines, primarily known in the pharmaceutical and food industries, with specific practices like Good Clinical Practice (GCP) and Good Pharmacovigilance Practice (GVP).
While GVP is less emphasized in EU legislation compared to Good Manufacturing Practice (GMP) and Good Distribution Practice (GDP), it is still essential for pharmacovigilance activities. The European Medicines Agency (EMA) is tasked with creating guidance on GVP, but there are no strict legal obligations for stakeholders to adhere to it, unlike GMP and GDP, which have clearer legal requirements.
The blog discusses the implications of these guidelines and suggests that GVP should be more clearly defined in legislation to enhance compliance and innovation in pharmacovigilance practices.
GxP in EU Legislation
There are dozens of different types of GxP and while other areas of good practices can be relevant to pharmacovigilance, for example Good Computer System Validation Practice (GCSVP) and Good Laboratory Practice (GLP), in this text we are looking at those that are mentioned in the European medicinal product (for human use) legislation.
GVP in EU Legislation
According to the GVP Introductory cover note, GVP was drawn up based on Article 108a(a) of Directive 2001/83/EC as amended, by the European Medicines Agency (EMA) in cooperation with the EU national competent authorities (NCA) and “interested parties” (it is unclear who these interested parties were).
The introductory cover note also states that:
Directive 2001/83/EC Article 108a states:
“In order to facilitate the performance of pharmacovigilance activities within the Union, the Agency shall, in cooperation with competent authorities and other interested parties, draw up:
(a) guidance on good pharmacovigilance practices for both competent authorities and marketing authorisation holders;
(b) scientific guidance on post-authorisation efficacy studies.”
Meaning that the EMA is legally required to write PV guidance and scientific guidance for PASS to facilitate PV activities in the EU. Based on our research, this EU legislative document, nor any other legal document, does not mention good pharmacovigilance practice in any other way.
The only legal requirement in the EU related to GVP is for the EMA to prepare the guidance, but there is no clear legal requirement for the Member States, marketing authorization holders, European Medicines Agency or the National Competent Authorities to follow it, unless you interpret that the part about facilitating performance of pharmacovigilance activities would mean that in order to perform the activities, the MAHs must follow GVP and that EMA is allowed to enforce the guidance they author as legal requirements.
However, if it is left to legal interpretation, why does the same legislative document refer much clearer to GMP, GCP, or GDP? And why do the GVP documents contain the explanatory part referring to legal requirements and recommendations?
GDP and GMP in EU Legislation
In comparison to the GVP reference in the EU legislation, let’s look at the wording for good distribution practice and good manufacturing practice in the same legal documents. They are described in the same part of Directive 2001/83/EC.
Article 46b
Article 47
“The Commission is empowered to adopt delegated acts in accordance with Article 121a in order to supplement this Directive by specifying the principles and guidelines of good manufacturing practices for medicinal products referred to in Article 46(f).”
Article 111
“The Commission is empowered to adopt delegated acts in accordance with Article 121a in order to supplement this Directive by specifying the principles and guidelines of good manufacturing practices for medicinal products referred to in Article 46(f).”
In short, the legislation says that the Member States must ensure that GMP and GDP are followed for active substances.
It is noteworthy that marketing authorization holders and the EMA have not been mentioned here. The legislation actually talks about the responsibilities of the Manufacturer, Manufacturing Authorization Holders and (national) competent authorities.
For example the Article 46 (f) states that the Manufacturer must at least:
“comply with the principles and guidelines of good manufacturing practice for medicinal products and to use only active substances, which have been manufactured in accordance with good manufacturing practice for active substances and distributed in accordance with good distribution practices for active substances.”
So we recognize that the below comparison of GvP requirements and its implications on marketing authorization holders isn’t exactly perfect, but it still tells an interesting story here.
GCP in EU Legislation
Somewhat similarly, the good clinical practice (GCP) is a different case in the scope of this blog post. GCP is not mentioned in the Directive 2001/83/EC because GCP is about the activities mainly performed before or outside of the scope of the manufacturing, distribution and providing the medicinal product to patients on the basis of the marketing authorization. In other words, GCP is applicable before there is a medicinal product.
GCP actually has a whole directive dedicated for its implementation: Directive 2001/20/EC.
Requirements for the conduct of clinical trials in the EU, including GCP and GMP and GCP or GMP inspections, are implemented in:
– Clinical Trial Directive (Directive 2001/20/EC)
– GCP Directive (Directive 2005/28/EC)
– Clinical Trials Regulation (Regulation 536/2014)
– Commission Implementing Regulation (Regulation 2017/556)
Side note
GMP also has its own piece of EU legislation that this text refers to later. In addition to being mentioned in the Directive 2001/83/EC, there is also a regulation and directive dedicated to GMP implementation supplementing the medicines directive. The directive explains in detail the purpose and legal requirements related to good manufacturing practices.
Referring to Legal Requirements in GVP
In the GVP text, any reference to Regulation 726/2004 and Directive 2001/83 refers to the Regulation and Directive respectively, always including their latest amendments.
Where reference is made to specific Articles in square brackets “REG” means Regulation 726/2004 and “DIR” means Directive 2001/83. Reference to specific Articles of the Implementing Regulation 520/2012 on the Performance of Pharmacovigilance Activities Provided for in Regulation 726/2004 and Directive 2001/83/EC is provided in square brackets with the indication “IR”.
If reference is provided to any other Regulation or Directive, its full reference is provided.
Should, May, Shall and Must in GVP
A keyword search was performed in the GVP documents simply by searching for “shall”, “should”, “must” and “may” and counted the total numbers. You can see the total numbers per each guideline at the bottom of this blog post.
The GVP documents contained the word “should” in total 2183 times and “shall” 371 times. Meaning that about 85% of sentences that contained either verb were recommendations. As mentioned, “may” and “must” were also used in the GVP documents and there the wording was even more heavily on the recommendations side.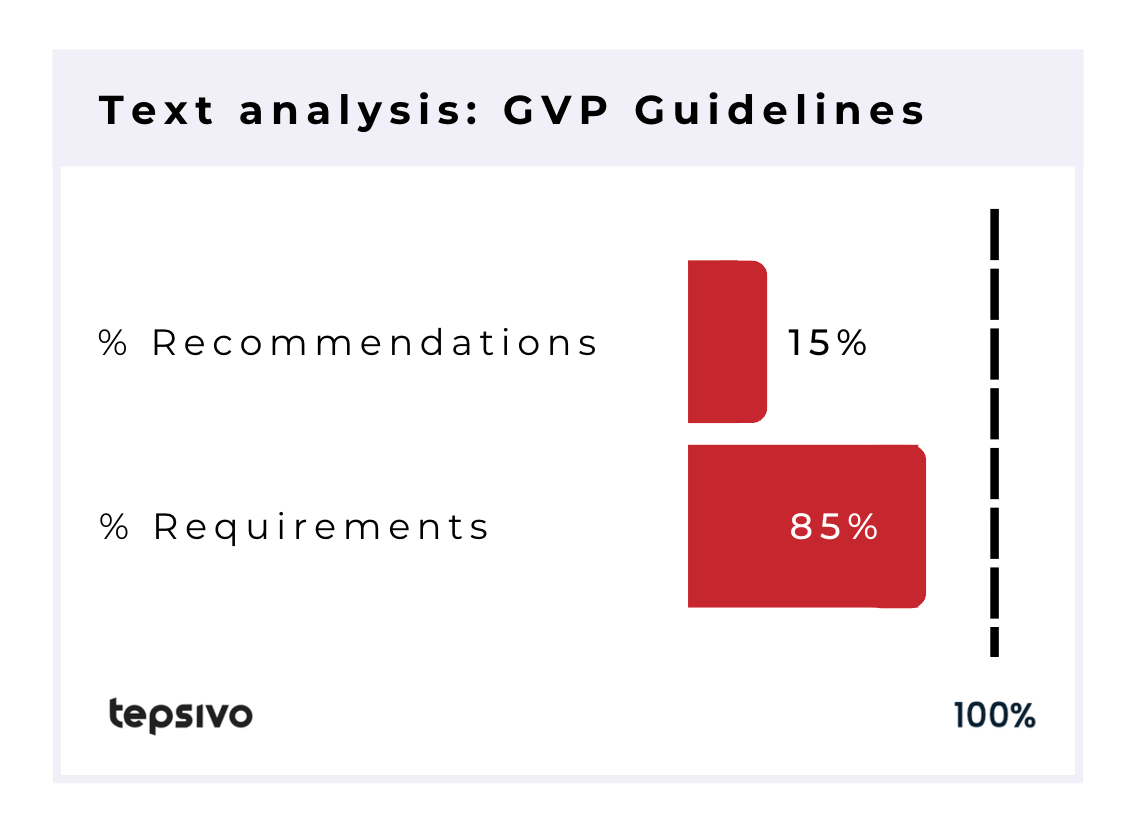
Shall, Should and Must in GMP
Good manufacturing practice (GMP) describes the minimum standard that a medicines manufacturer must meet in their production processes. In the EU the EMA coordinates inspections performed by the competent authorities to verify compliance of the manufacturers with these standards and plays a key role in harmonizing GMP activities.
The EU Directive 2017/1572 states:
Wording-wise the GMP documents contain 94% recommendations, however for the reasons described above, these recommendations can be considered to be requirements that have to be met. The GMP also has a legislative part in the EU, where 100% of the wording is with “shall” and is even more clearly legal requirements.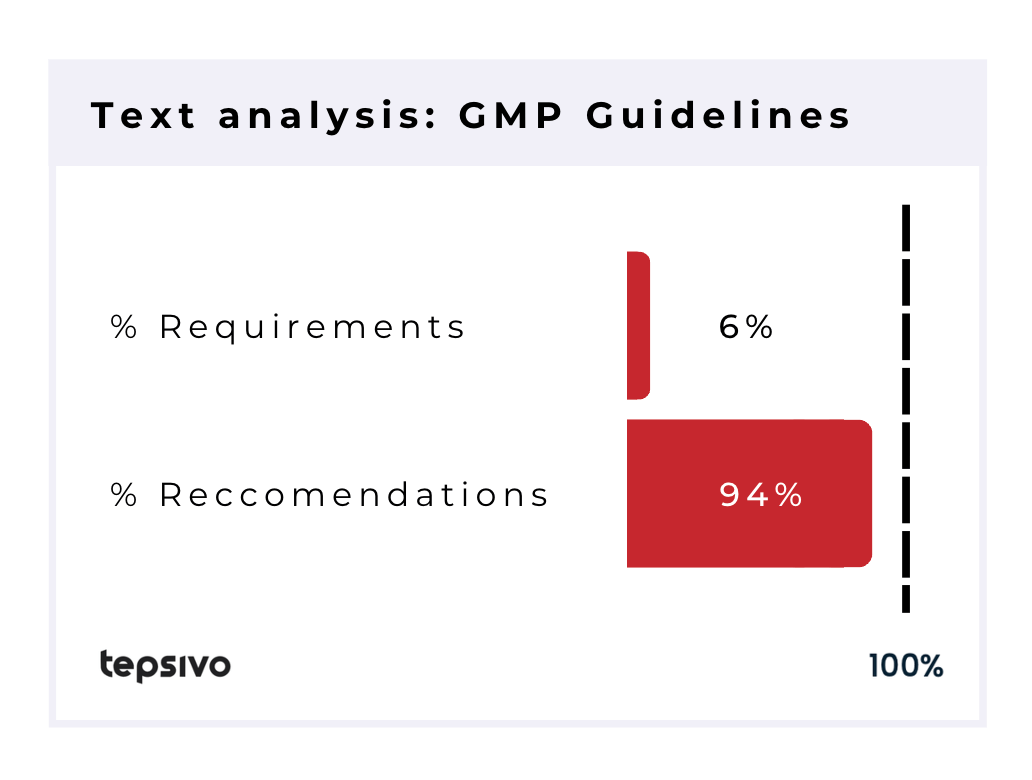
Shall, Should and Must in GDP
Good distribution practice (GDP) describes the minimum standards that a wholesale distributor must meet to ensure that the quality and integrity of medicines is maintained throughout the supply chain.
The two GDP guidelines contain “should” 343 times and “shall” only 2 times, but “may” and “must” are used as well, so there’s about 92% recommendation type of wording in the GDP documents.
Again, as shown above, it is clear from the legislation that good distribution practices are legally required to be followed.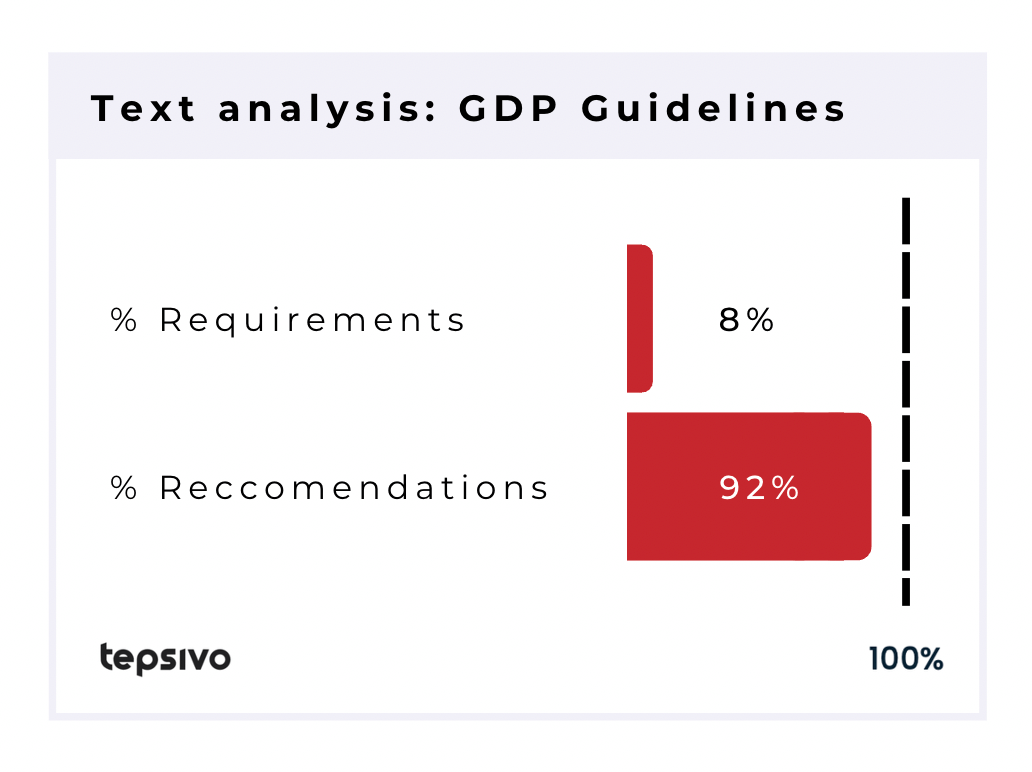
Shall, Should and Must in GCP
Good clinical practice (GCP) is an international ethical and scientific quality standard for designing, recording and reporting trials that involve the participation of human subjects. Compliance with this standard provides public assurance that the rights, safety and wellbeing of trial subjects are protected and that clinical-trial data are credible.
A number of documents in EudraLex – Volume 10 – Clinical trials guidelines have been revised and updated to bring them in line with the changes required by the Clinical Trials Regulation 536/2014. Additionally, new documents were prepared to cover new aspects introduced by the same Regulation.
During the transitional period, which will last until 30 January 2025, both sets of documents (old and new) will apply accordingly and should be referred to respectively according to the legislation under which the Clinical trial is conducted. Here we look only at the new set of documents.
The GCP documents contain mainly (97%) recommendation types of wording, but it should be interpreted that the texts are legally enforceable.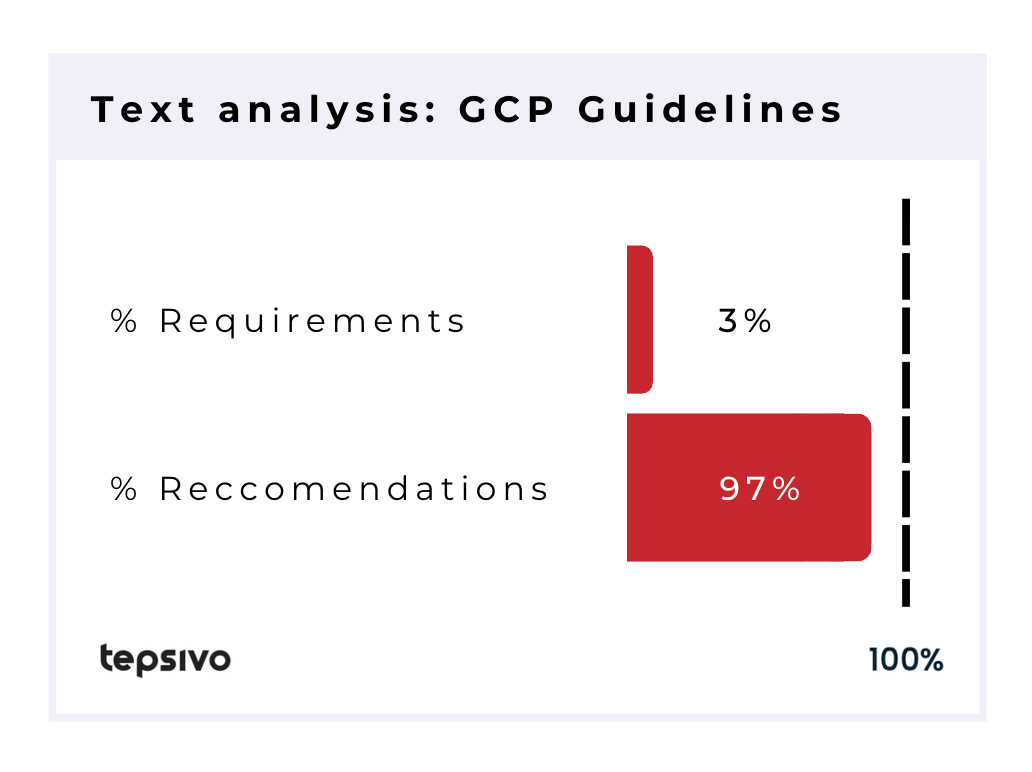
Legal Interpretation
Only about 15% of the contents of GVP refer to legal requirements. While many are aware of this fact, the traditional wisdom has been to read all the recommendations in the GVP as if they were legal requirements in the hopes of a successful result in pharmacovigilance inspection. Such approach hinders innovation.
One of the main reasons why Tepsivo was founded was to shift away from this trend of blindly following what everyone else is doing and adding process upon process based on comments from auditors and inspectors, who do not necessarily make a difference between legislation and guidelines.
Meanwhile the directive for GMP implementation, for example, explains that the goal is to have a clear set of rules that can be followed by the manufacturers and the inspectors from the competent authorities. But the wording in GMP is also in recommendation format.
The EU directive for medicinal products refers to the responsibility of the EMA to prepare guidance on good pharmacovigilance practices for both competent authorities and marketing authorisation holders in order to facilitate the performance of pharmacovigilance activities.
The medicinal products legislation describes that it is the responsibility of the EU Member states to take appropriate measures to ensure that the manufacture, import and distribution of active substances on their territory comply with good manufacturing practice and good distribution practices for active substances.
The implementation of GMP and good clinical practice in the conduct of clinical trials on medicinal products for human use are described in their own separate legislations.
How should this be interpreted?
One overly simplified interpretation could be that following GVP is not legally required (except for the parts that refer to legal requirements and are covered by the legislation) and following GCP, GMP and GDP is legally required, even if the text is written to sound like a recommendation.
Another interpretation could be that the fact that EU GVP is written by the EMA to facilitate the PV activities in the EU due to a legal requirement, implementation of GVP by MAHs and EMA is similarly a legal requirement as implementation of GCP, GMP and GDP by the relevant parties.
Third perspective could be that even though there is legislation in place describing the implementation of GCP, GMP and GDP, their contents can be interpreted similarly as the requirements and recommendations in GVP.
Most likely none of these is the correct interpretation, and it would not be a surprise if there was no clear answer out there.
Side note
It is important to notice that the GVP and maintenance of PV system in the EU is mainly the responsibility of the EMA, the national competent authorities and the marketing authorization holders, but the responsible parties in GCP, GMP and GDP are often not the MAH or the EMA, but the manufacturer and competent authority.
What Should be Done?
Should GVP, in full, be legally required the same way as GCP, GMP and GDP? Should you follow GVP fully, even though it is not mandatory?
One point of view could be that the wording related to GVP should be clarified and GVP should be clearly required by the legislation. The reasoning behind this is that most of the stakeholders already completely follow the GVP and take the contents of the GVP guidelines as the word of the law, the same way as the other GxP.
Making implementation of GVP legally required in the similar fashion as the other GxP mentioned in this blog post would also require that relevant directive and regulation are put in place for the implementation of GVP and the update of the actual GVP documents, but after this adjusting the expectations related to pharmacovigilance activities would be more flexible to update compared to the current situation.
In the current situation, following GVP makes sense in large parts, however the documents do contain some out-of-date and/or vague recommendations which have led to practices in the industry that have ballooned the administrative tasks around pharmacovigilance making it an area that can be very expensive with little to no value to the understanding of the product’s benefit-risk profile.
Another way to go would be to highlight even more, if possible, that GVP contains mainly recommendations for the pharmaceutical companies and the regulators and that these documents should be taken as such (recommendations) both in the day-to-day PV activities and in the inspections conducted by the regulatory authorities.
This is probably how many of the quality experts see the good practice guidelines: the role of the guidelines is to interpret the law and be helping the companies and regulators complying with the law, mainly because laws are often written in a complex legal jargon which one can not assume everyone implementing the processes in companies understands fully. As such, it can include recommendations that, again, should be helpful but do not necessarily need to be followed, and required parts which you must follow. If you don’t follow the recommendations, then you bear the burden of proving that you comply with the law otherwise.
Strictly sticking with those pharmacovigilance legal requirements that are in the legislation and in GVP with “shall” wording could remove or significantly reduce significantly quite a few activities from your PV system that most PV professionals consider to be cornerstones of the PV system. This would also lead to more varied interpretations of the legislation and different kinds of PV systems. There is potential that companies would be able to create truly innovative PV systems extremely well suitable for their products, the kind Tepsivo offers. On the flip side it is also possible that companies would not perform the safety-efficacy monitoring sufficiently, or at all.
So, best to keep GVP the way it is, mainly a set of recommendations with room to innovate to meet legal requirements. But it’s worth clearly specifying to the industry what in fact is legally required and where you can fit the PV system for purpose.
Side note
It is important to notice that the GVP and maintenance of PV system in the EU is mainly the responsibility of the EMA, the national competent authorities and the marketing authorization holders, but the responsible parties in GCP, GMP and GDP are often not the MAH or the EMA, but the manufacturer and competent authority.
Annexes
Should GVP, in full, be legally required the same way as GCP, GMP and GDP? Should you follow GVP fully, even though it is not mandatory?
One point of view could be that the wording related to GVP should be clarified and GVP should be clearly required by the legislation. The reasoning behind this is that most of the stakeholders already completely follow the GVP and take the contents of the GVP guidelines as the word of the law, the same way as the other GxP.
Making implementation of GVP legally required in the similar fashion as the other GxP mentioned in this blog post would also require that relevant directive and regulation are put in place for the implementation of GVP and the update of the actual GVP documents, but after this adjusting the expectations related to pharmacovigilance activities would be more flexible to update compared to the current situation.
In the current situation, following GVP makes sense in large parts, however the documents do contain some out-of-date and/or vague recommendations which have led to practices in the industry that have ballooned the administrative tasks around pharmacovigilance making it an area that can be very expensive with little to no value to the understanding of the product’s benefit-risk profile.
Another way to go would be to highlight even more, if possible, that GVP contains mainly recommendations for the pharmaceutical companies and the regulators and that these documents should be taken as such (recommendations) both in the day-to-day PV activities and in the inspections conducted by the regulatory authorities.
This is probably how many of the quality experts see the good practice guidelines: the role of the guidelines is to interpret the law and be helping the companies and regulators complying with the law, mainly because laws are often written in a complex legal jargon which one can not assume everyone implementing the processes in companies understands fully. As such, it can include recommendations that, again, should be helpful but do not necessarily need to be followed, and required parts which you must follow. If you don’t follow the recommendations, then you bear the burden of proving that you comply with the law otherwise.
Strictly sticking with those pharmacovigilance legal requirements that are in the legislation and in GVP with “shall” wording could remove or significantly reduce significantly quite a few activities from your PV system that most PV professionals consider to be cornerstones of the PV system. This would also lead to more varied interpretations of the legislation and different kinds of PV systems. There is potential that companies would be able to create truly innovative PV systems extremely well suitable for their products, the kind Tepsivo offers. On the flip side it is also possible that companies would not perform the safety-efficacy monitoring sufficiently, or at all.
So, best to keep GVP the way it is, mainly a set of recommendations with room to innovate to meet legal requirements. But it’s worth clearly specifying to the industry what in fact is legally required and where you can fit the PV system for purpose.
Table 1: Good Pharmacovigilance Practice
| Should | May | Shall | Must | Requirements | Recommendations |
| 92 | 21 | 30 | 1 | 22% | 78% |
Module II – Pharmacovigilance system master file (Rev 2)
| Should | May | Shall | Must | Requirements | Recommendations |
| 97 | 48 | 41 | 16 | 28% | 72% |
Module III – Pharmacovigilance inspections (Rev 1)
| Should | May | Shall | Must | Requirements | Recommendations |
| 29 | 59 | 15 | 1 | 15% | 85% |
Module IV – Pharmacovigilance audits (Rev 1)
| Should | May | Shall | Must | Requirements | Recommendations |
| 53 | 5 | 12 | 1 | 18% | 82% |
Module V – Risk management systems (Rev 2)
| Should | May | Shall | Must | Requirements | Recommendations |
| 210 | 64 | 11 | 2 | 5% | 95% |
| Should | May | Shall | Must | Requirements | Recommendations |
| 702 | 101 | 72 | 7 | 9% | 91% |
Module VI Addendum I – Duplicate management of suspected adverse reaction reports
| Should | May | Shall | Must | Requirements | Recommendations |
| 80 | 24 | 1 | 3 | 4% | 96% |
Module VII – Periodic safety update report (Rev 1)
| Should | May | Shall | Must | Requirements | Recommendations |
| 347 | 82 | 82 | 0 | 16% | 84% |
Module VIII – Post-authorisation safety studies (Rev 3)
| Should | May | Shall | Must | Requirements | Recommendations |
| 103 | 85 | 56 | 1 | 23% | 77% |
| Should | May | Shall | Must | Requirements | Recommendations |
| 6 | 0 | 4 | 0 | 40% | 60% |
Module IX – Signal management (Rev 1)
| Should | May | Shall | Must | Requirements | Recommendations |
| 103 | 39 | 14 | 0 | 9% | 91% |
| Should | May | Shall | Must | Requirements | Recommendations |
| 23 | 29 | 0 | 2 | 4% | 96% |
Module X – Additional monitoring
| Should | May | Shall | Must | Requirements | Recommendations |
| 22 | 4 | 8 | 0 | 24% | 76% |
Module XV – Safety communication (Rev 1)
| Should | May | Shall | Must | Requirements | Recommendations |
| 109 | 27 | 16 | 0 | 11% | 89% |
Module XVI – Risk minimisation measures: selection of tools and effectiveness indicators (Rev 2)
| Should | May | Shall | Must | Requirements | Recommendations |
| 161 | 67 | 9 | 0 | 4% | 96% |
Module XVI Addendum I – Educational materials
| Should | May | Shall | Must | Requirements | Recommendations |
| 46 | 0 | 0 | 0 | 0% | 100% |
Totals for Good Pharmacovigilance Practice
| Should | May | Shall | Must | Requirements | Recommendations |
| 2183 | 655 | 371 | 34 | 15% | 85% |
Table 2: Good Manufacturing Practice
GMP Guide Chapter 1 Pharmaceutical Quality System
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 23 | 1 | 0 | 2 | 8% | 92% |
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 37 | 6 | 2 | 8 | 19% | 81% |
GMP Guide Chapter 3 Premises and Equipment
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 61 | 3 | 0 | 4 | 6% | 94% |
GMP Guide Chapter 4 Documentation
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 67 | 10 | 0 | 4 | 5% | 95% |
GMP Guide Chapter 5 Production
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 131 | 10 | 2 | 4 | 4% | 96% |
GMP Guide Chapter 6 Quality Control
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 84 | 5 | 0 | 2 | 2% | 98% |
GMP Guide Chapter 7 Outsourced Activities
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 21 | 2 | 0 | 5 | 18% | 82% |
GMP Guide Chapter 8 Complaints, Quality Defects and Product Recalls
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 71 | 14 | 0 | 0 | 0% | 100% |
GMP Guide Chapter 9 Self Inspection
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 6 | 1 | 0 | 0 | 0% | 100% |
Total numbers for Good Manufacturing Practice
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 478 | 51 | 4 | 27 | 6% | 94% |
Legislative part of Good Manufacturing Practice
Legislation Pharmaceutical Quality System
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 0 | 0 | 1 | 0 | 100% | 0% |
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 0 | 0 | 9 | 0 | 100% | 0% |
Legislation Premises and Equipment
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 0 | 0 | 3 | 0 | 100% | 0% |
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 0 | 0 | 11 | 0 | 100% | 0% |
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 0 | 0 | 6 | 0 | 100% | 0% |
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 0 | 0 | 7 | 0 | 100% | 0% |
Legislation Outsourced Activities
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 0 | 0 | 6 | 0 | 100% | 0% |
Legislation Complaints, Quality Defects and Product Recalls
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 0 | 0 | 4 | 0 | 100% | 0% |
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 0 | 0 | 2 | 0 | 100% | 0% |
Totals for the legislative part of Good Manufacturing Practice
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 0 | 0 | 49 | 0 | 100% | 0% |
Table 3: Good Distribution Practice
Guidelines on Good Distribution Practice of medicinal products for human use
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 245 | 11 | 1 | 29 | 10% | 90% |
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 98 | 3 | 1 | 1 | 2% | 98% |
Totals for Good Distribution Practice
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 343 | 14 | 2 | 30 | 8% | 92% |
Table 4: Good Clinical Practice
Template statement on compliance Regulation (EU) 2016/679
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 0 | 0 | 0 | 0 | 0% | 0% |
Compensation for trial participants
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 1 | 3 | 0 | 0 | 0% | 100% |
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 13 | 6 | 2 | 2 | 17% | 83% |
Investigator Curriculum Vitae template
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 2 | 1 | 0 | 0 | 0% | 100% |
Declaration of interest template
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 1 | 1 | 0 | 0 | 0% | 100% |
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 4 | 2 | 0 | 1 | 14% | 86% |
Informed consent and patient recruitment procedure template
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 12 | 6 | 0 | 0 | 0% | 100% |
Compliance with applicable rules for biological samples
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 3 | 3 | 1 | 1 | 25% | 75% |
ICH guideline E2F – Note for guidance on development safety update reports
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 141 | 1 | 0 | 0 | 0% | 100% |
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 30 | 4 | 0 | 0 | 0% | 100% |
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 0 | 0 | 0 | 0 | 0% | 0% |
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 120 | 23 | 9 | 6 | 9% | 91% |
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 4 | 2 | 0 | 0 | 0% | 100% |
Union Basic Format for Manufacturer’s Authorisation
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 2 | 0 | 1 | 0 | 33% | 67% |
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 328 | 42 | 1 | 3 | 1% | 99% |
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 191 | 44 | 1 | 3 | 2% | 98% |
Auxiliary medicinal products in clinical trials
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 16 | 16 | 9 | 2 | 26% | 74% |
Guidance for the conduct of good clinical practice inspections (August 2017)
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 15 | 8 | 0 | 1 | 4% | 96% |
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 29 | 6 | 1 | 1 | 5% | 95% |
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 5 | 4 | 0 | 0 | 0% | 100% |
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 5 | 4 | 0 | 0 | 0% | 100% |
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 24 | 5 | 0 | 0 | 0% | 100% |
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 10 | 3 | 1 | 2 | 19% | 81% |
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 5 | 2 | 0 | 0 | 0% | 100% |
Guidance for the preparation of good clinical practice inspections (August 2017)
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 16 | 21 | 0 | 2 | 5% | 95% |
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 15 | 9 | 3 | 0 | 11% | 89% |
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 33 | 13 | 1 | 0 | 2% | 98% |
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 4 | 1 | 0 | 3 | 38% | 63% |
Guideline on reporting serious breaches
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 66 | 31 | 3 | 4 | 7% | 93% |
Appendix III b – Information to be submitted with a notification of a serious breach Accelerating clinical trials in the EU (ACT EU) – Delivering an EU clinical trials transformation initiative
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 8 | 0 | 0 | 0 | 0% | 100% |
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 173 | 36 | 0 | 2 | 1% | 99% |
Guideline for good clinical practice – ICH E6(R2) – EMA/CHMP/ICH/135/1995
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 365 | 55 | 0 | 0 | 0% | 100% |
Draft ICH E6 (R3) Guideline on good clinical practice (GCP) – Step 2b
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 550 | 106 | 0 | 0 | 0% | 100% |
Risk proportionate approaches in clinical trials
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 67 | 34 | 3 | 0 | 3% | 97% |
Summaries of Clinical Trial Results for Laypersons
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 31 | 20 | 0 | 2 | 4% | 96% |
Good Lay Summary Practice Guidance (GLSP)
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 225 | 140 | 5 | 22 | 7% | 93% |
Ethical considerations for clinical trials on medicinal products conducted with minors
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 136 | 22 | 19 | 0 | 11% | 89% |
Total numbers for Good Clinical Practice
| Should | May | Shall | Must | Requirements |
Recommendations
|
| 2650 | 674 | 60 | 57 | 3% | 97% |
Did you like the article? Share with your network!
…or tell us your opinion.
Follow our newsletter!
Keep up with industry trends and get interesting reads like this one 1x per month into your inbox.
Learn more about Tepsivo
We deliver modern PV solutions to fulfill your regulatory needs using less resources. See how we do it >

0 Comments