Pharmacovigilance in Argentina
How is it with drug safety system in “the Land of Silver”? Follow our guide and you will be in the picture.
And of course, if you feel you need a local consultant, we’re here for you.
Current status of our Argentine pharmacovigilance services:
Argentina’s Contact Person
AVAILABLE
Easily managed through Tepsivo Platform >
Local Literature Screening
AVAILABLE
Automated monitoring with Tepsivo Literature >
Who is the main PV authority in Argentina?
Interesting website sections related to pharmacovigilance
Other interesting sections related to pharmacovigilance
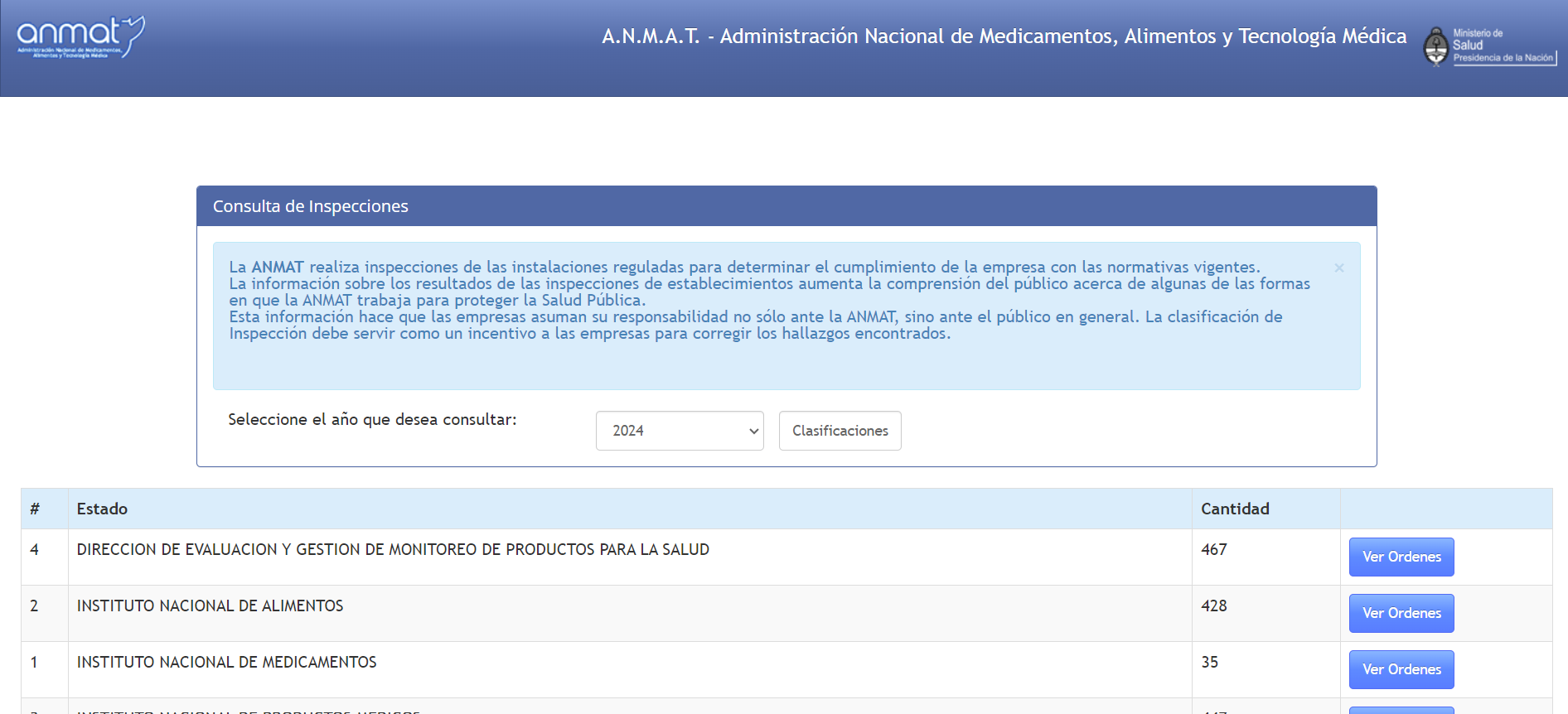
If you are interested in annual data by the ANMAT pharmacovigilance department in the document format, look here (even though the most recent years are missing).
All important forms in one place.
Other important institutions & industry groups
Some of the most important laws
Local pharmacovigilance specifics
Useful abbreviations to know
If you want to study local materials about pharmacovigilance in Argentina, following terms will be helpful for you to get familiar with:
SNFVG (Sistema Nacional de Farmacovigilancia)
→ National pharmacovigilance system
RAM (Reacción Adversa a Medicamento)
→ ADR / Adverse Drug Reaction
RFV (Responsable de Farmacovigilancia)
→ QPPV / Qualified Person for Pharmacovigilance
PGR (Plan de Gestión de Riesgos)
→ RMP / Risk Management Plan
IPAS (Informes Periódicos de Actualización de Seguridad)
→ PSUR / Periodic Safety Update Report
TAD platform (Trámites a distancia)
→ national platform for remote procedures (online reporting to authorities)
Is Qualified Person Responsible for Pharmacovigilance needed?
How can be adverse events reported in Argentina?
Adverse reaction reports can be submitted using online forms that are available on available on this ANMAT subpage.
The forms differ for healthcare professionals, patients and pharmaceutical companies. Reporting by email or a special portal is also possible (well described on the linked page).
Local medical journals to screen
Here are few examples of Argentine local literature that medical authorization holders should monitor:
LA PRENSA MEDICA ARGENTINA
One of the world’s most renowned journals in the field of medicine, published since 1914.
Publishes original research, reviews, commentaries, short communications, and editorials.
ISSN: 0032-745X
REVISTA ARGENTINA DE CIRUGÍA
The official scientific publication of the Argentine Association of Surgery (Asociación Argentina de Cirugía).
Aims to disseminate original contributions to knowledge in the field of surgery.
ISSN: 0048 – 7600
REVISTA ARGENTINA DE SALUD PÚBLICA
Already mentioned in the section about Argentine institutions, this is a journal published by the Ministry of Health (MSAL), so definitely good to put this authoritative literature on your screening list too.
ISSN: 1853-810X
REVISTA CIENTÍFICA ANMAT
A journal edited by ANMAT, published since 2017. Focused mainly on regulators of health products.
ISSN: 2796-7646
Rather then spending your budget for hours of repetitive manual work to monitor those sources, we recommend to simply automate this task using our Tepsivo Literature solution.
Other interesting resources
Book: Good Pharmacovigilance Practices in Latin America
Manual de Buenas Prácticas de Farmacovigilancia edición Latinoamérica
A free pdf to download with over 700 pages, linked also by ANMAT on their website. If you feel you would appreciate an extensive resource about pharmacovigilance not only in Argentina, but the overall region of Latin America, this is a book you might like.
Articles about regulatory requirements and policies
Pharmacovigilance Regulatory Requirements in Latin America
An article that analyses pharmacovigilance regulatory requirements across 21 countries in Latin America.
Pharmaceutical Policy in Argentina
A chapter about pharmaceutical industry and its regulations in Argentina.
Studies related to PV in Argentina
A study from Argentina that investigated the level of knowledge about pharmacovigilance and the activity of reporting an adverse event among local HCP, including dentists.
As mentioned in this analysis, University of San Luis was designated as “Peripheral Effector of the National Pharmacovigilance System” – the document is focused on ADR reports received there between years 2010 and 2013.
Adverse drug reactions as a reason for admission to an internal medicine ward in Argentina
Results from screening of 1045 patients, where probable ADR accounted for about 10% of admissions to an internal medicine ward.
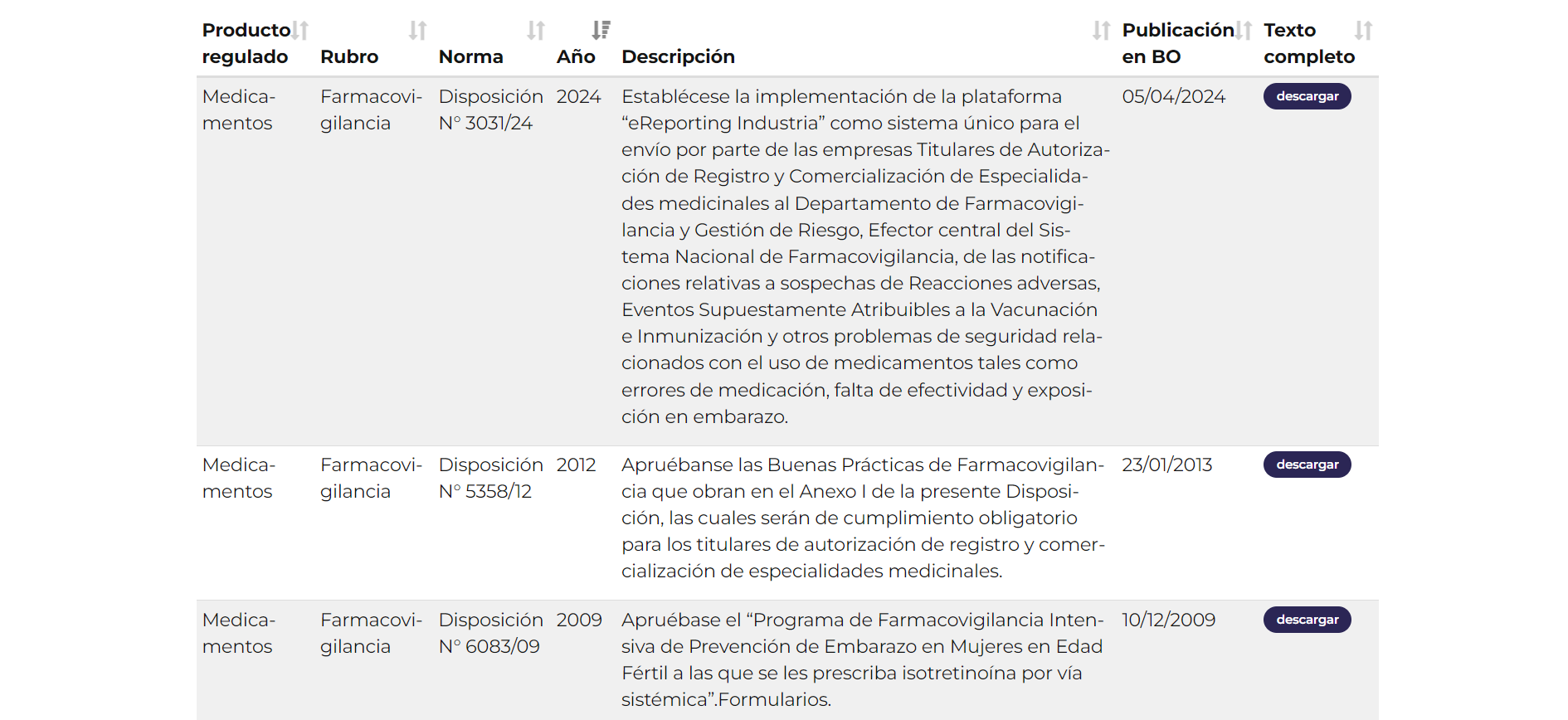
RMP, PSUR and other documents related to reporting
If you plan a submission of RMP in Argentina, you can download related forms on this page.
Just as the previous link leads you tu the page related to RMP documents, this one lists PSUR sheets for download.
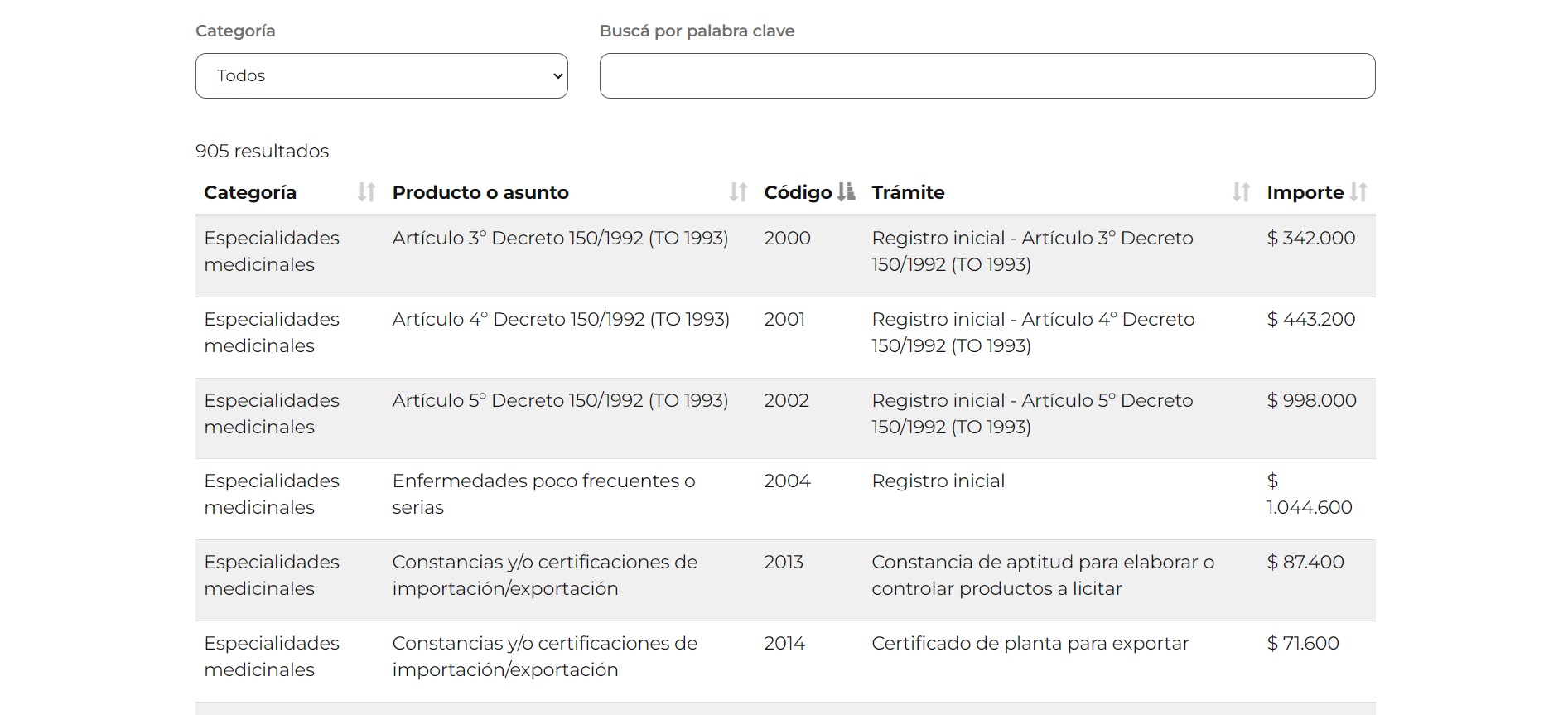
A table related to PSUR reporting, available in the forms section of ANMAT website.
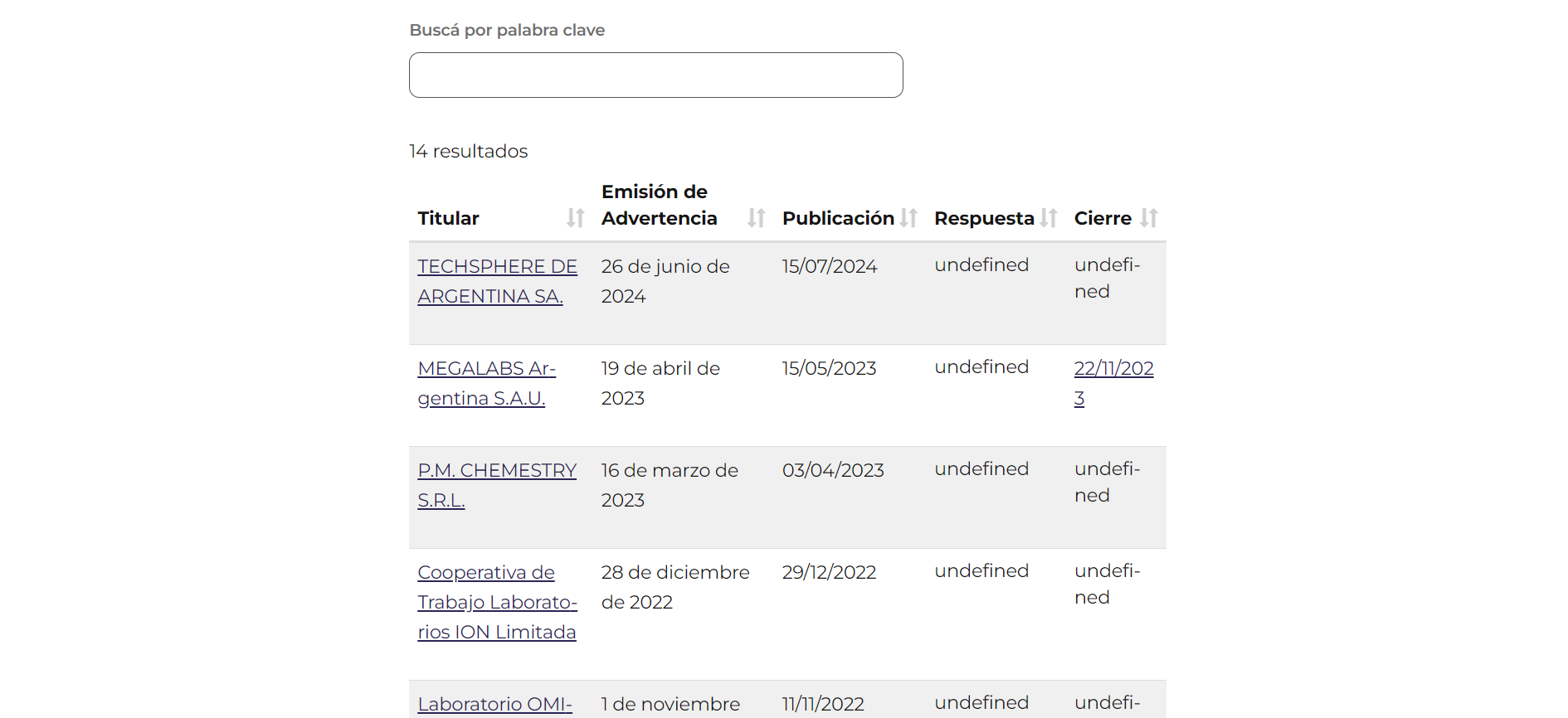
A table related to ADRs detection, also from the forms section of ANMAT website.
Do you need some help with putting the Argentine PV System together? We’re here for you.

And while you’re here…
…consider also our global end-to-end PV solution!
Thanks to the Tepsivo platform, you can get a full pharmacovigilance system in all countries you need – for a fraction of the usual budget.

Tepsivo Oy | Urho Kekkosen katu, 4-6 E, 00 100, Helsinki, Finland | VAT number FI31367614 | contact@tepsivo.com | +358 402 204 698 | Privacy policy