No time to read now?
-> Download the article as a handy pdf
List of contents
Signal detection performed by pharmaceutical companies should stop
Why the EU pilot program is the right step forward?
Martti Ahtola | Mar 14, 2022
In other words, for around 95% of the APIs and 99% of the products, EudraVigilance does not have to be monitored by the MAHs for the purpose of signal detection. The pilot program is a right step towards one major change in European pharmacovigilance requirements.
It seems, however, that this has been missed by many in the industry as we can see most companies and PV providers still continue to perform zero value-added activities (daily downloads of ICSRs, quarterly signal management reports, etc.). We suspect that the main reason for this is the unclear and very limited communication from the EMA related to signal detection, not just related to the pilot phase but across the board. Plus, of course, as we know, the traditionally sluggish PV organizations are unable to react to changes quickly enough.
As a company pushing pharmacovigilance into what can be called as value-based healthcare, we always strongly advocate that any activities which add no value, have zero impact on patient safety, and in this case are not even required by enforcers of applicable legislation, should not be performed. And signal detection performed by MAH in EudraVigilance is definitely one of those activities (save for those products on the EMA pilot list).
This blog post provides further detail on this position, explains the current requirements, and publicly makes the call to all PV organizations who this can affect and who still perform needless tasks to stop, help decrease costs on PV, and allow scientists time to do work that matters.
We also argue that given the negligible amount of data potentially relevant for patient safety that is not reported to EudraVigilance, it is worth considering whether the MAHs should be asked to perform signal detection independently of centralized analyses.
Background to the EU pilot
EU pharmacovigilance legislation states that MAHs shall continuously monitor data in EudraVigilance to the extent of their access to the database with a frequency proportionate to the identified risk, the potential risks and the need for additional information and to inform EMA and National Competent Authorities (NCAs) of validated signals detected in the database. These legal requirements for MAH signal detection and reporting are applicable to all medicinal products authorised in the European Union without distinction and their full implementation may cause duplication of signal detection activities, as well as a high volume of signal notifications by MAHs to EMA and NCAs, which will have to be processed within the legal timeline of 30 days. The EU signal detection pilot was started to streamline the implementation of the requirements to continuously monitor the authority database and inform regulatory authorities of validated signals. According to EMA, the signal detection pilot is part of phased implementation of signal detection requirements. The phased implementation was agreed with the European Commission (EC) to reduce the “risk of overloading the EU network with signals from MAHs, while gaining experience with the new process.” One of the goals of the EU signal detection pilot was to perform informed impact assessment of involvement of MAHs in the monitoring of EudraVigilance and give insight into:- Resource implications
- Important safety issues detected
- EV tools and areas of guidance and process to be further clarified or streamlined
- Duplication of signals for substances with several MAHs
Situation before the pilot
EMA employees and Pharmacovigilance Risk Assessment Committee (PRAC) members have published an article about the first six years of signal detection activities (2012 – 2018). The article reviews the first 6 years of the EU signal detection and management process by the EU network for centrally and nationally authorized products.
According to the article, 26,848 potential signals were reviewed by EMA’s signal management team. They reviewed in-depth information on:
- 13,550 potential signals (i.e., drug-event pairs from screening of the EV database, scientific literature, or information received from other regulatory authorities, etc.),
- 563 signals were validated by the team,
- 465 signals were confirmed by the PRAC and
- 453 recommendations were given by the PRAC of which more than half were for drug labelling changes (an update of the product information).
Other recommendations during this period included routine pharmacovigilance and monitoring within the periodic safety update report (34%), referral evaluations (3.8%), or updates to risk management plans (2%).
This means that less than 2% of all signals reported to, EMA led to any kind of recommendation. Or if phrased differently: more than 98% of the validated signals were either incorrectly assessed by the MAHs or they were duplicate reports.
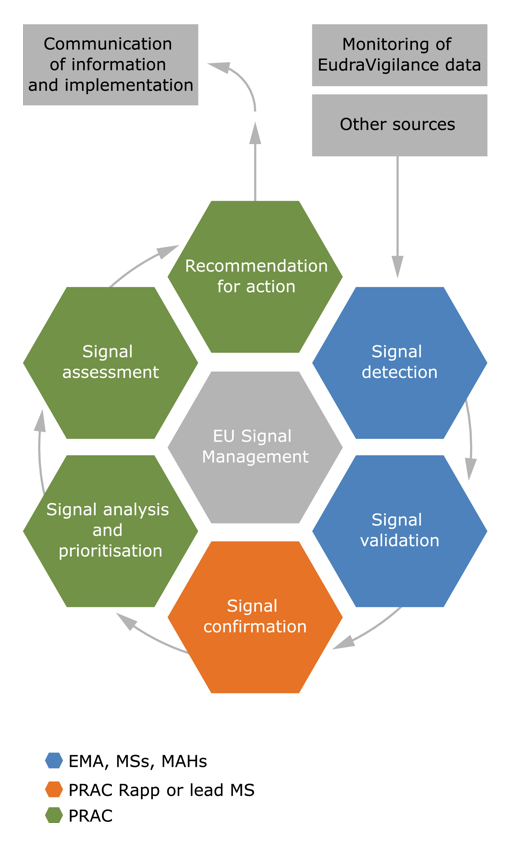
EMA signal management process, MS = Member State
Current situation
Currently, the EMA website states that the pharmaceutical companies whose products are not part of the EU Signal Pilot Program have no obligation to continuously monitor EudraVigilance and inform the regulatory authorities of validated signals from EudraVigilance. In practice, this means that downloading cases or line listings from EV continues to be unnecessary for most of the products. We have previously referred to the (lack of) usefulness of signal management by the MAHs and will continue doing so, until a sensible solution is found. The transitional period was supposed to be over by the end of 2021. The pilot program started in February 2018 and the transitional arrangements have been now extended twice. The legislation on pharmacovigilance activities is outdated as it still requires the marketing authorization holders to continuously monitor EudraVigilance. Without updated legislation, the pilot phase for signal management is likely to continue beyond 2022. Why? This is probably because, under the current legislation, the only direction EMA could go is to implement the requirements to apply to all MAHs and by now, it is probably clear to the EMA and EC that in practice signal monitoring by pharmaceutical companies brings no additional information about the benefit-risk profile of products, but comes with high additional burden of incorrectly assessed and duplicate signals.Results of the pilot
In October 2018 EMA announced that the signal pilot phase is postponed until end of 2019 mainly due to need of more information and expected lack of resources after Brexit. The pilot phase was extended until end of 2019 and before the end of the pilot, in September 2019 EMA was scheduled to deliver a “report on the first year of experience, including both workload and process aspects, and this will be used as the basis to agree and communicate on the next implementation phase, including the scope of products to be included and date of coming into effect.” No information about the report nor the decision is available on EMA website, on European Commission’s publications repository or register of commission documents, but EMA’s annual activity report for 2019 contains the following information: “Information of the operation of the pilot was collected during the pilot in Q1/Q2 to support a report to the European commission in Q3. The report was submitted to the EC. The EC has decided to extend the pilot for 24 months until the end of 2021.” In order to see this report and decision, we have submitted a document request to the European Commission. EMA’s annual activity report for 2020 states the following: “Based on the analysis of the pilot of MAH signal detection in EudraVigilance, implement the decision of the European Commission on extension beyond the pilot phase, including new business process for MAH signals.” and “Activity reduced to pilot phase. The pilot was extended by EC until December 2021 and it is currently ongoing (6 notifications in 2020, including 2 valid but not confirmed). Business process for MAH signals delivered in November 2019 (post-EC Internal Audit Service audit action).” EMA’s mid-year report for 2021 states that the action related to the signal detection pilot for EMA is to “Together with stakeholders, develop new and improved continuous surveillance and signal detection methodology using the network’s pharmacovigilance database” with the goal to develop a “Guidance for surveillance and signal detection” and for “Enhanced communication with the network”. In the same report, EMA declared the following achievements for the first half of 2021: “Specific guidance and system specifications were developed with the expert groups and discussed at several stakeholder meetings during the first part of the year. Signal management process development and work share proposals are ongoing.” Also worth mentioning is that EMA had been planning to start a project during Q4 2021 on Signal and Safety Analytics to “increase saleability and efficiency in processing of signals & safety data”. One of the goals of this project for 2022 is “Collection of IT and business requirements for the design of the new EudraVigilance data analysis system (EVDAS) platform / electronic Reaction Monitoring Reports (eRMR) solution / ADR website”.Results of Signal Management Process
In March 2019 the annual EudraVigilance report stated that EMA had reviewed 2,204 potential signals in 2018 and 78.7% of these signals originated from monitoring the EudraVigilance database. Other signals were generated from clinical studies and scientific literature. 1,800 of these potential signals were not validated and were immediately closed. In other words, 81.7% of the potential signals reported to EMA were immediately discarded as incorrect. In 2018, PRAC assessed 74 safety signals and the Member States 40. That is, only 3.4% of all reported potential signals were assessed in more detail. 50 of these signals assessed resulted directly in a recommendation to change the product information, 6 of these cases resulted in recommended direct healthcare professional communications (DHPC), 2 resulted also in recommended update of RMP and 1 signal led to the update of RMP to fully characterise and investigate the concern. The EMA’s annual activity report for 2020 shows that the pilot program might have resulted to a small reduction in the number of false signals reported by the MAHs and number of validated signals.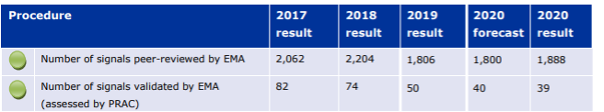
Number of signal reports reviewed by EMA and number of signals validated by PRAC 2017 – 2020
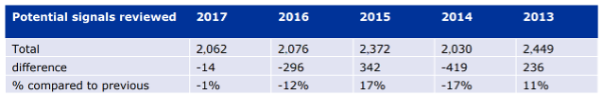
Number of potential signals reported to EMA 2013 – 2017
Why MAHs should not be doing signal detection?
The amount of this data that is available to the MAH varies based on the products that they have. If the MAH has a generic product that is commonly used, they are likely to receive large amounts of reports and they will likely identify many articles that mention their product or the active pharmaceutical ingredient (API) used in their product. However, the benefit-risk profile of these generic and widely used products is well known and truly new information (valid signals) are rarely identified. If the product is new and innovative, it is likely that new information for the benefit-risk profile is received. However, the number of the reports will most likely be low as most of the new innovative products are aimed at small group of patients. The current “recommended” methods for signal validation are only suitable for situations where there is a large amount of patients and ADR reports. If the large number of patients and reported adverse events are missing, we are no longer talking about signal detection but clinical assessment of individual cases. To reduce this problem, the guidelines recommend that the MAHs take into consideration also cases from similar products and treatments. This means that the MAH should monitor also reports from other companies and other products. Why should this be the responsibility of the pharmaceutical company, when there is a party (or parties) who has easy access to all this data and is being paid by all the pharmaceutical companies to perform this activity?What really should be done?
At this point of the text, it should be clear to the reader that the signal detection should be only the responsibility of the authorities who have the access to the largest amount of data. The best way to detect signals would be to truly centralize signal detection. This would happen by delivering the data from the local authority databases to one central database. Currently the best option would be WHO’s VigiBase where most of the ICSRs reported to the regulatory authorities are already going. This way would lead to significantly lower duplication by the local regulatory authorities and pharmaceutical companies, more relevant data available for detecting potential signals, and one set of methods for detection. VigiBase contains data from the 149 Members of the WHO Programme for International Drug Monitoring. VigiBase is maintained and developed by the Uppsala Monitoring Center (UMC) and members of the WHO Programme for International Drug Monitoring (PIDM) can access and analyze this common resource using VigiLyze, a signal detection and management tool provided by UMC. There is already regular screening taking place to find previously unrecognized or incompletely documented suspected adverse drug reactions (ADRs). According to the UMC website “the signal detection process has to rely on a combination of computerised data-mining methodology for selection of medicine-adverse effect combinations, and subsequent clinical evaluation of reports by members of the medical and scientific team.”Conclusion
The amount of additional information on potential signals outside of what is reported to EMA that can be found by individual MAHs is extremely limited. And, as we have shown, there is no point for MAHs to review the same dataset as what the central European authority analyzes (EudraVigilance).
With this in mind, there are steps forward that the industry can take to remove unnecessary burden and, of course, costs.
First, the MAHs should assess whether their existing pharmacovigilance processes are effective and whether they really perform activities that may impact patient safety and are required by law. They should focus on removing any duplication of effort that only ties up valuable experts’ time and significantly increases costs. Modern, streamlined process driven by technology is the right way to go.
Second, while EMA deserves credit for the pilot program and centralizing signal detection activities, it would be helpful for the agency to communicate the real consequences and highlight that MAHs simply do not need to perform certain activities that had, for no good reason, become standard (e.g. daily ICSR downloads from EV).
Third, legislators should come back to the pharmacovigilance law and update it. There is arguably a lot more to update but this one is low-hanging fruit and it should be supported by the EMA and any other stakeholders.
Did you like the article? Share with your network!
…or tell us your opinion.
Follow our newsletter!
Keep up with industry trends and get interesting reads like this one 1x per month into your inbox.Learn more about Tepsivo
We deliver modern PV solutions to fulfill your regulatory needs using less resources. See how we do it >

0 Comments