5+ Examples of AI in Pharmacovigilance
No more theory. Real use cases with artificial intelligence available today.
Author: Martti Ahtola
Last update: 10.6.2025
Use case #1
AI for medical literature monitoring
If you asked us what benefits come from directly integrating artificial intelligence into a literature screening tool like Tepsivo Literature, here’s our answer: “Next-level precision and increased time savings”.
But you probably want a little more detail, right?
Evolution of literature surveillance
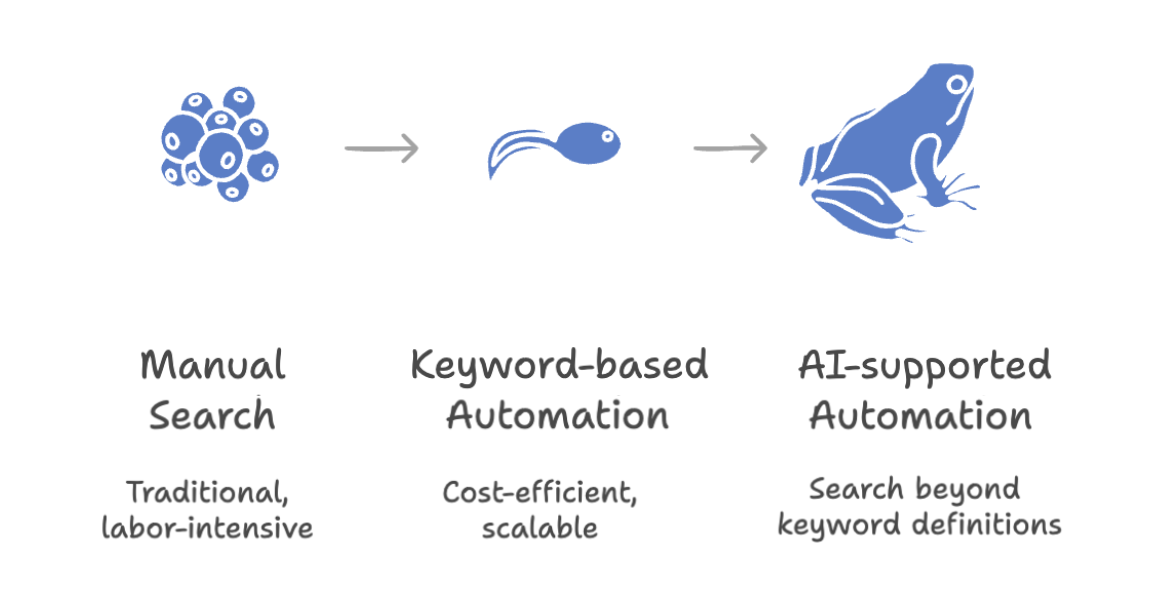
First, make no mistake — we’re not just talking about improvement over the traditional manual screening process.
Even compared to modern, fully automated literature monitoring solutions, the AI features bring a significant edge.
Improved search & smart assessment
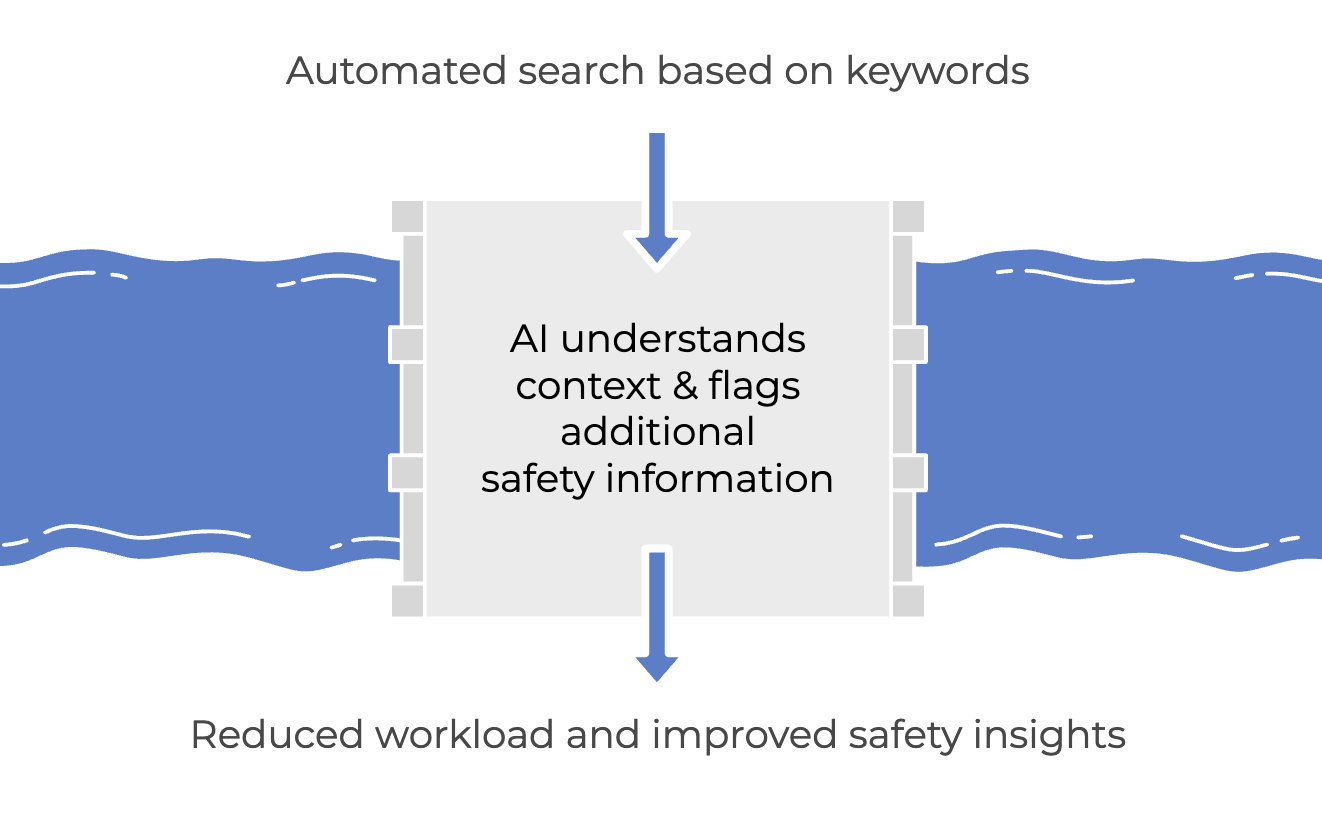
Using artificial intelligence, article search no longer relies on defined keywords alone. The AI understands context, acting as an extra control layer.
It can flag safety-relevant info that’s tricky or impossible to spot with standard rule-based automation, directing pharmacovigilance experts straight to what matters and saving their time.

The AI is not another tool – it is fully baked into the interface of modern literature automation solutions (that’s the case with our Tepsivo Literature). This is how it looks in practice:

Screenshot: AI assessment of an article from a scientific journal (includes explanation of the status)
And this is still not the best part. We kept our favourite benefit for the end: with the built-in artificial intelligence, safety reports can be automatically generated based on the articles!
Sounds good, right?
And did you know our solution easily monitors tens of thousands of non-indexed scientific journals for as low as €100 / month per country?
Use case #2
AI-assisted imports
to safety database
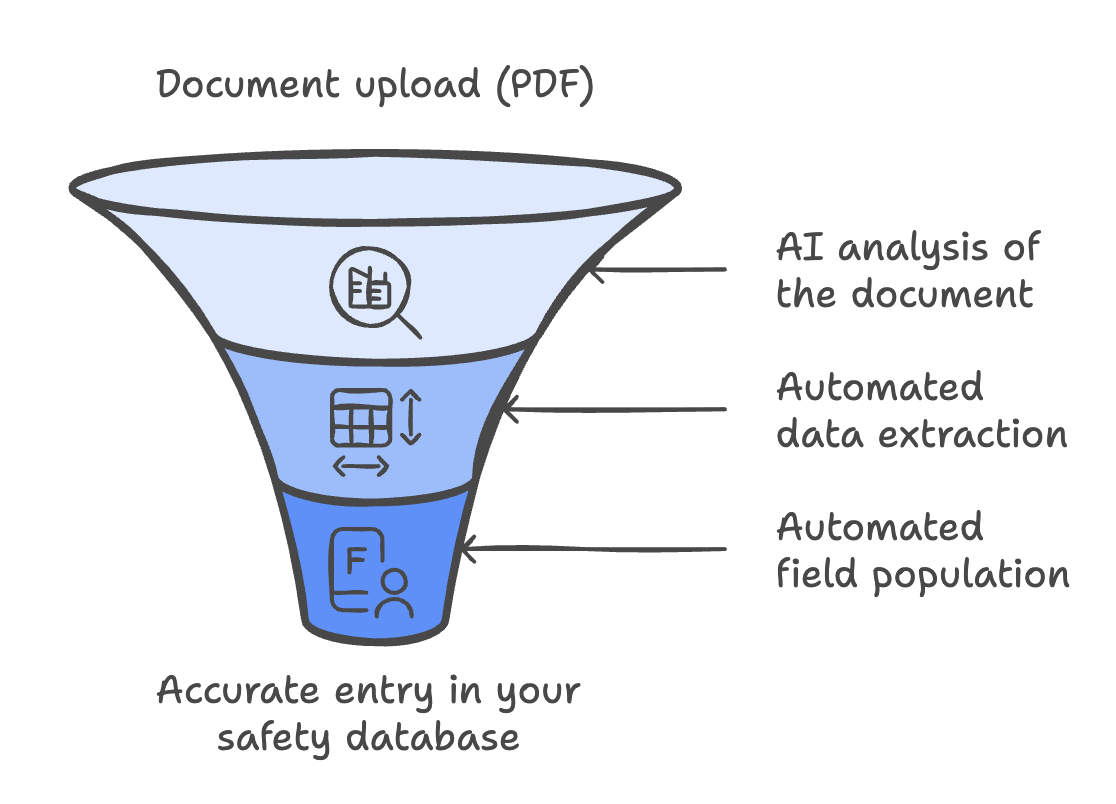
Picture this: you upload a PDF with case reports or other documents into your safety database…
…and the AI built in the software automatically pulls out key info about adverse events, reporter details, and more — then fills in the right fields for you.
That’s exactly what artificial intelligence in the most modern safety databases can do!
Artificial intelligence handling the imports understands the uploaded content and processes it better than simple automation.
We can tell you from our own experience that this really saves a lot of time for PV experts, especially with longer documents.
Is it expensive, you ask? Doesn’t have to be. You can actually get a safety database with AI features (and no ICSR limits!) starting at €1,000 per month — check our pricing if you’re curious.
But not to forget — this isn’t limited to PDF uploads.
The same AI-assisted process can be applied to safety reports from any source:
Video: Automated data upload from Safety Reporting to Tepsivo Safety Database using AI capabilities of Tepsivo OnePV
If all parts of your PV system are interconnected (it’s 2025, they should be…), you can populate fields in the safety database literally with a click of a button when sending a report to authority is needed.
Tepsivo
OnePV
If you haven’t come across an all-in-one solution like this one yet, it might be a good time to take a look at our Tepsivo OnePV >>
Tepsivo
OnePV
If you haven’t come across an all-in-one solution like this one yet, it might be a good time to take a look at our Tepsivo OnePV >>
Use case #3
AI-powered automated translation
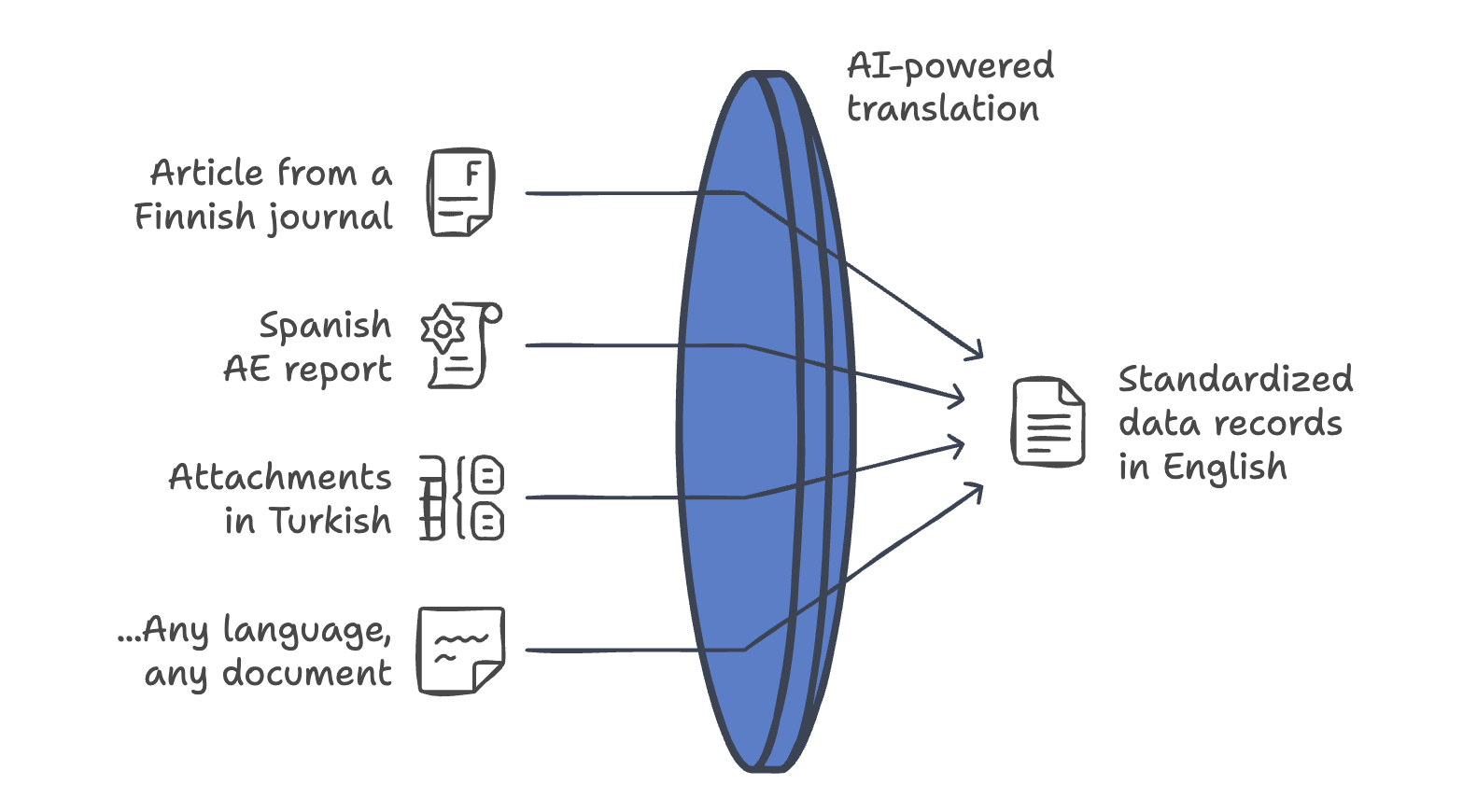
Imagine receiving a safety report in a native language or an article from a local medical journal — say it’s Portuguese — and having it seamlessly translated into clean, well-written English without ever leaving your system and needing third-party software.
This means faster workflows, fewer errors from manual copy-pasting, and consistent terminology across all your reports and documents.
A little side note here: Many don’t realize it, but even online translation tools like Google Translate are powered by AI these days. If you rely on such solutions in your processes, still be sure to keep the “AI classification” in mind when it comes to pharmacovigilance SOPs for authority inspections.
But back to more advanced use of AI translation – directly integrated in pharmacovigilance systems. When we say that artificial intelligence speeds up and improves tasks like automated literature search and safety reporting, the AI-driven translation is in fact a key part of these workflows in modern pharmacovigilance software. See below!
Video: AI-powered translation of a safety report in Tepsivo OnePV
The automated translation using AI makes global safety processes really smooth – no matter what languages come your way. More about the way we do safety reporting >>
The automated translation using AI makes global safety processes really smooth – no matter what languages come your way.
Use case #4
Narrative writing done by AI
What takes up a lot of time in pharmacovigilance is drafting case narratives. But AI gives a hand.
The AI technology integrated within our Tepsivo Safety Database also assists in drafting the “Narrative Case Summary” — quickly producing a focused, clear, and factual description of the case.
Instead of starting from scratch, you get a solid draft to review and finalize, saving time and cutting down repetitive work. The artificial intelligence picks up key details and even includes the specific words or short phrases used by the reporter, helping keep the narrative accurate and true to the original information.
You can also define simple rules – what the narrative should include and how it should be structured. That way, the AI adapts to your company’s style and regulatory requirements, ensuring consistency and compliance.
Video: Using the AI narrative writing feature in Tepsivo Safety Database
Use cases #5, 6, 7…
More ways how AI helps Tepsivo customers
Automated case intake with AI features
Fully integrated with subsequent steps of safety reporting in one place
In-app AI assistant tailored to each customer
Giving quick, direct answers based on SOPs / product documentation etc.
AI chatbot for regulatory intelligence
Delivers explanation of regulatory-related queries in realtime
Automated case intake with AI features
Fully integrated with subsequent steps of safety reporting in one place
In-app AI assistant tailored to each customer
Giving quick, direct answers based on SOPs / product documentation etc.
AI chatbot for regulatory intelligence
Delivers explanation of regulatory-related queries in realtime
We’re sure you get the message: with the right tools, areas of your pharmacovigilance system that AI significantly improves are many. Contrary to vague conference talks and overpriced “AI courses”, we believe the future is already here – and it’s simple to quickly leverage the technology in your drug safety processes. Let us help you with that!
Let’s have a chat
Whatever your needs are, we look forward to getting in touch with you.
Feel free to drop us a message and we will contact you right away.
Tepsivo Oy | Haartmaninkatu 4, Building 14, 00290, Helsinki, Finland | VAT number FI31367614 | contact@tepsivo.com | +358 402 204 698 | Privacy policy